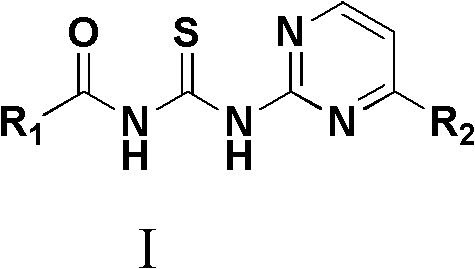
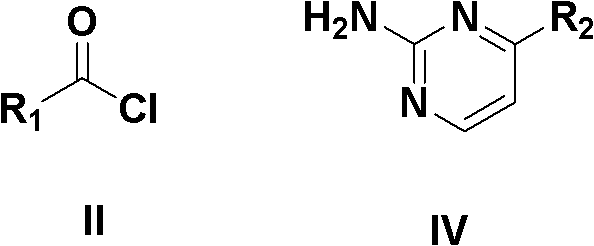
N-substituted pyridine acyl-N-substituted pyrimidyl thiourea derivant as well as preparation and application thereof
A technology of pyrimidinyl thiourea and pyridine acyl group, which is applied to N-substituted pyridine acyl-N-substituted pyrimidinyl thiourea derivatives and the fields of preparation and application thereof, and can solve problems such as strong toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] Example 1: Preparation of N-pyrimidinyl-N-nicotinoylthiourea
[0047] In a 100mL round bottom flask, add 0.97g (10mmol) of NH in a solution of 10mmol of nicotinoyl chloride dissolved in 20mL of dichloromethane 4 PEG-600 solution of dichloromethane in NCS (10mL, including 9.5mL of dichloromethane and 0.5mL of PEG-6000), stirred and refluxed for 15min, left to cool, and filtered with suction to obtain an orange solution, which was added with 10mmol 2 - A solution of aminopyrimidine dissolved in 5 mL of anhydrous acetonitrile, stirred at room temperature for 10 h, allowed to stand overnight, filtered with suction, and the filter cake was recrystallized with a mixed solvent of DMF and water at a volume ratio of 1:1 to obtain the product.
[0048] Yellow solid, yield 42.3%, melting point 185-186°C; 1 H NMR (DMSO-d 6 )δ: 7.21-9.06 (m, 6H, Py and ph), 12.26 (s, 1H, NH), 13.36 (s, 1H, NH); IR / cm -1 : 3420(N-H), 3167(N-H), 1718(C=O), 1554, 1452(Ar), 1263(C=S); ESI-MS: 258; El...
example 2
[0049] Example 2: Preparation of N-4-methylpyrimidinyl-N-nicotinoylthiourea
[0050] In a 100mL round bottom flask, add 0.97g (10mmol) of NH in a solution of 10mmol of nicotinoyl chloride dissolved in 20mL of dichloromethane 4 NCS dichloromethane PEG-600 solution (10mL, including 9.5mL dichloromethane, PEG-6000.5mL), stirred and refluxed for 15min, left to cool, and suction filtered to obtain an orange solution, which was added with 10mmol of 2-amino - A solution of 4-methylpyrimidine dissolved in 5 mL of anhydrous acetonitrile, stirred at room temperature for 10 h, allowed to stand overnight, filtered with suction, and the filter cake was recrystallized with a mixed solvent of DMF and water at a volume ratio of 1:2 to obtain the product.
[0051] Yellow solid, yield 55.83%, melting point 192-194°C; 1 H NMR (DMSO-d 6 )δ: 2.43(s, 3H, CH 3 ), 7.22-9.15 (m, 6H, Py and ph), 11.88 (s, 1H, NH), 13.98 (s, 1H, NH) IR / cm -1: 3417(N-H), 3125(N-H), 1703(C=O), 1550, 1450(Ar), 1261(C=S...
example 3
[0052] Example 3: Preparation of N-pyrimidinyl-N-2-chloronicotinoylthiourea
[0053] In a 100mL round bottom flask, add 0.97g (10mmol) of NH in a solution of 10mmol of 2-chloronicotinyl chloride dissolved in 20mL of acetonitrile 4 NCS dichloromethane PEG-600 solution (10mL, including 9.5mL dichloromethane, PEG-6000.5mL), stirred and refluxed for 15min, left to cool, and suction filtered to obtain an orange solution, which was added with 10mmol of 2-amino Dissolve pyrimidine in 10 mL of anhydrous acetonitrile, stir at room temperature for 10 h, let stand overnight, filter with suction, take the filter cake and recrystallize with a mixed solvent of DMF and water at a volume ratio of 2:1 to obtain the product.
[0054] Yellow solid, yield 65.87%, melting point 190-191°C; 1 H NMR (DMSO-d 6 )δ: 7.22-8.98 (m, 6H, Py-H and ArH,), 12.34 (s, 1H, NH), 13.21 (s, 1H, NH); IR / cm -1 : 3425(N-H), 3157(N-H), 1720(C=O), 1548, 1450(Ar), 1264(C=S); ESI-MS: 292; Elemental analysis for C 11 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com