Synergistic effect of arteannuim and derivative thereof on chemotherapeutic agent
An artemisinin derivative and artemisinin technology, applied in the medical field, can solve the problem of no reports of artemisinin and its derivatives being combined with chemotherapeutic agents to treat tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
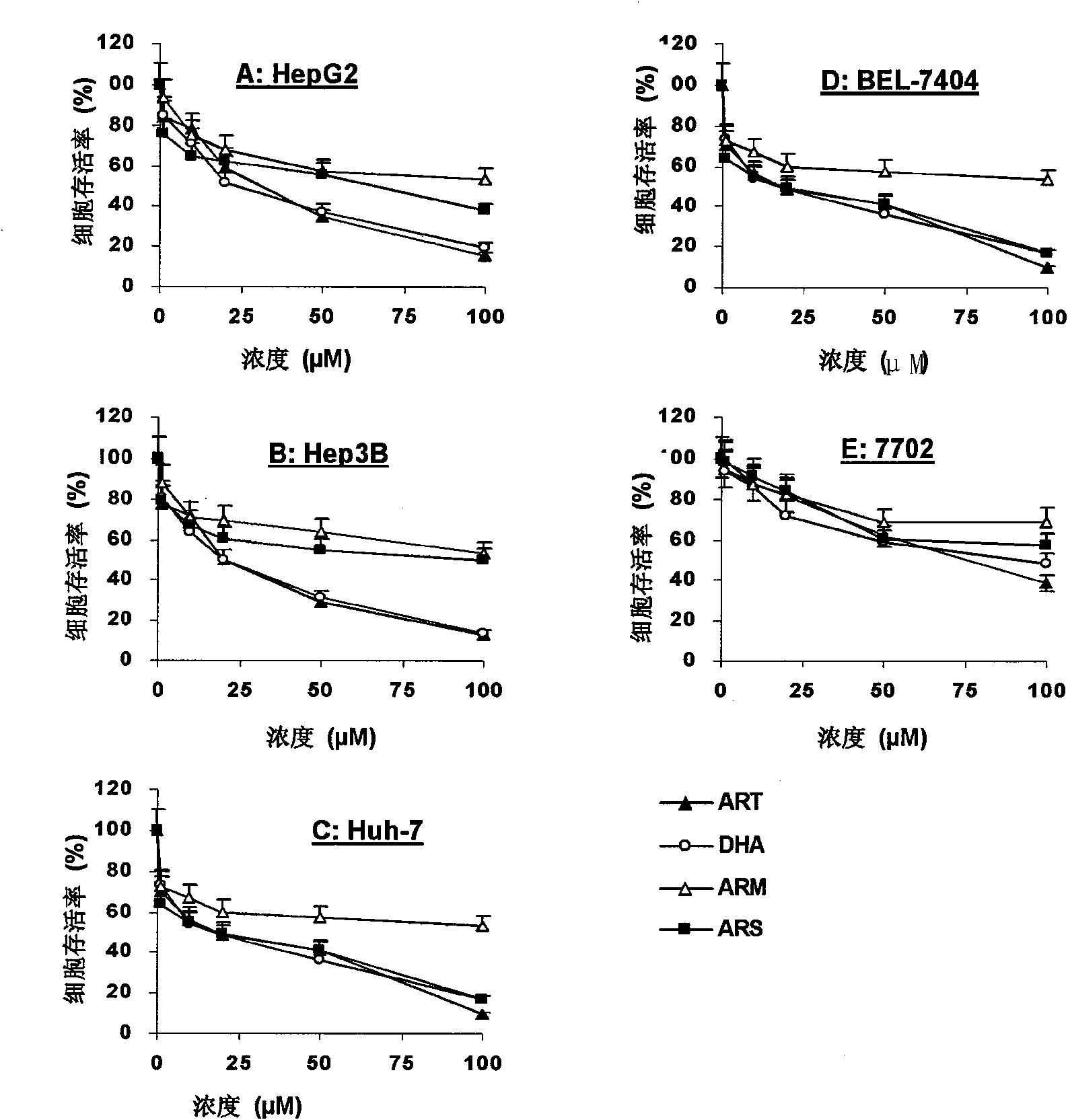
[0041] Example 1 The killing effect of artemisinin and its derivatives on liver cancer cells
[0042] In this experiment, four liver cancer cell lines with different p53 genetic backgrounds were used for drug activity screening in vitro, namely HepG2 cells expressing wild-type p53, Hep3B cells lacking p53, and BEL-7404 and Huh-7 cells with p53 mutations ( Cell Bank of Type Culture Collection Committee of Chinese Academy of Sciences / Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences), for artemisinin (ART), dihydroartemisinin (DHA), artemether (ARM) and artesunate (ARS) (Zhejiang Yiwu Gaodeng Fine Chemical Co., Ltd.) was screened for its cytotoxicity.
[0043] Four artemisinin compounds: ART, DHA, ARM and ARS acted on human liver cancer cells HepG2 ( figure 1 A), Hep3B ( figure 1 B), Huh-7( figure 1 C), BEL-7404 ( figure 1 D) and normal human liver cells 7702 ( figure 1 E). Cells were seeded in 96-well plates, and different conc...
Embodiment 2
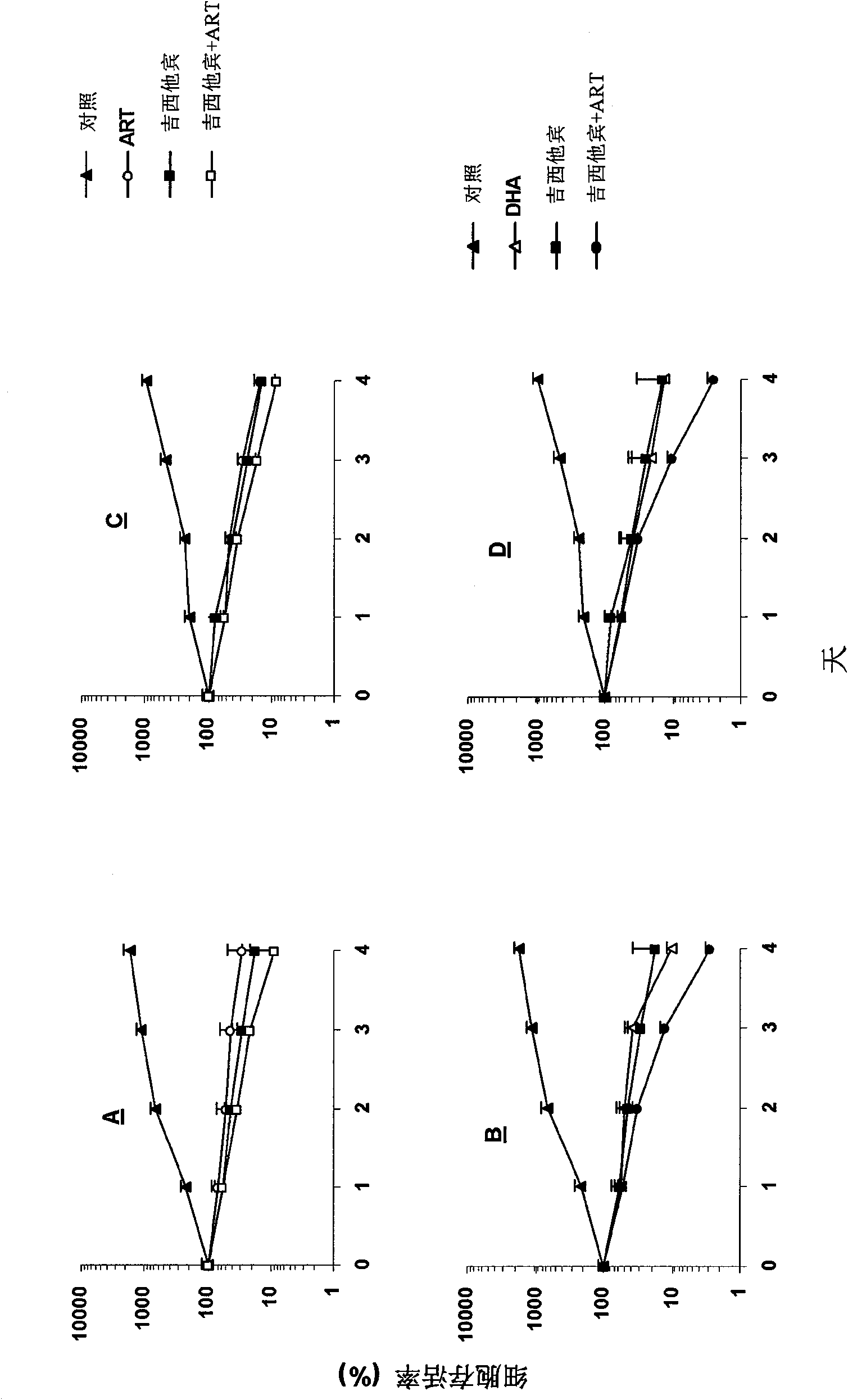
[0050] Example 2 Inhibition of artemisinin and its derivatives combined with gemcitabine (gemcitabine) on the growth of human liver cancer cells
[0051] As shown in Example 1, artemisinin and dihydroartemisinin alone in vitro can significantly inhibit the proliferation of liver cancer cells. We tested whether they have chemosensitizing effects. Artemisinin and dihydroartemisinin Combined with gemcitabine to act on human liver cancer cells HepG2 and Hep3B respectively, and observe the inhibitory effect of combined drugs on cell growth.
[0052] HepG2 cells were separately treated with 10 μmol / L ART (A) and 10 μmol / L DHA (B) for different time (0, 24, 48, 72, 96 hours), then added MTT, read the OD value of different action time, and plotted Cell growth curve, Hep3B cells were treated with 10 μmol / L ART (C) and 10 μmol / LDHA (D) under the same conditions. 10 μmol / L artemisinin and dihydroartemisinin combined with 10 μg / L gemcitabine were applied to HepG2 and Hep3B cells (0, 24, ...
Embodiment 3
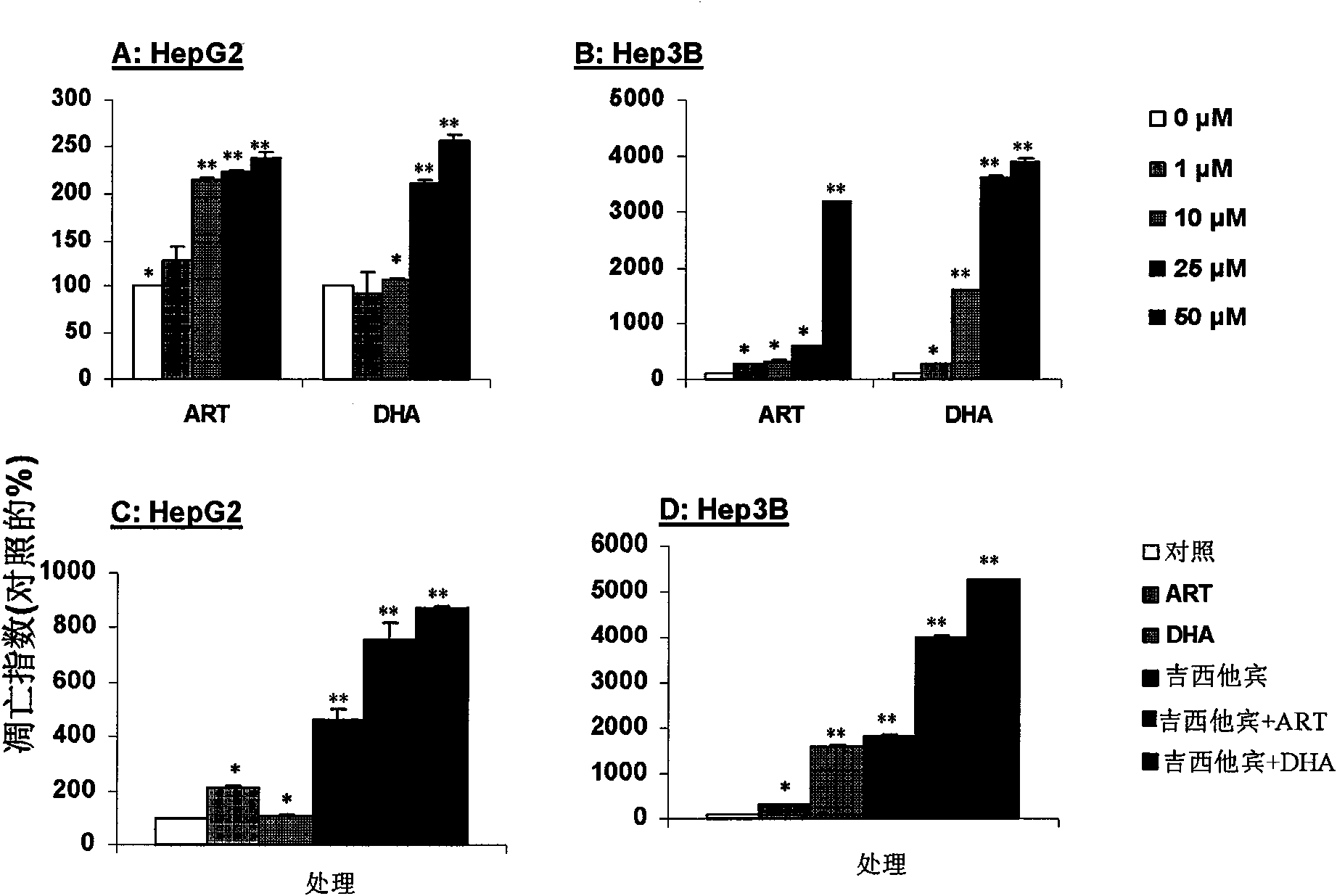
[0060] Example 3 Artemisinin and dihydroartemisinin combined with gemcitabine induce apoptosis of human liver cancer cells
[0061] The inventors studied the relationship between the inhibitory effect of artemisinin and dihydroartemisinin on the growth of human liver cancer cells and apoptosis, the specific experiments are as follows. The result is as image 3 Shown:
[0062]Artemisinin and dihydroartemisinin induce apoptosis of human liver cancer cells HepG2 (A) and Hep3B (B): HepG2 and Hep3B cells in logarithmic growth phase were treated with 0, 1, 10, 25, 50 μmol / L Treat with artemisinin or dihydroartemisinin for 48 hours, collect adherent cells and dead cells, perform Annexin V / PI double staining, quantitatively detect the proportion of apoptotic cells by flow cytometry, and merge early apoptotic cells with late apoptotic cells As an assessment of apoptosis index.
[0063] Synergistic effect of artemisinin and dihydroartemisinin on gemcitabine: HepG2 (C) and Hep3B (D) c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com