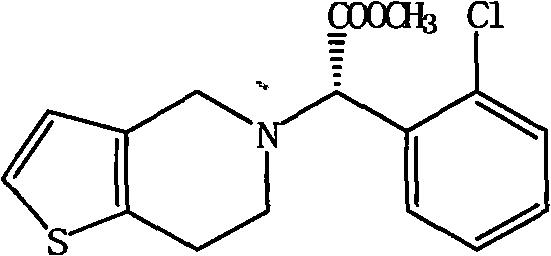
Method for improving resolution yield of clopidogrel camphorsulfonate
A technology of clopidogrel camphorsulfonate and camphorsulfonate, which is applied in the field of synthesis to improve the yield of dexclopidogrel camphorsulfonate, and can solve the problem of complex resolution steps and low yield of clopidogrel resolution and other issues, to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
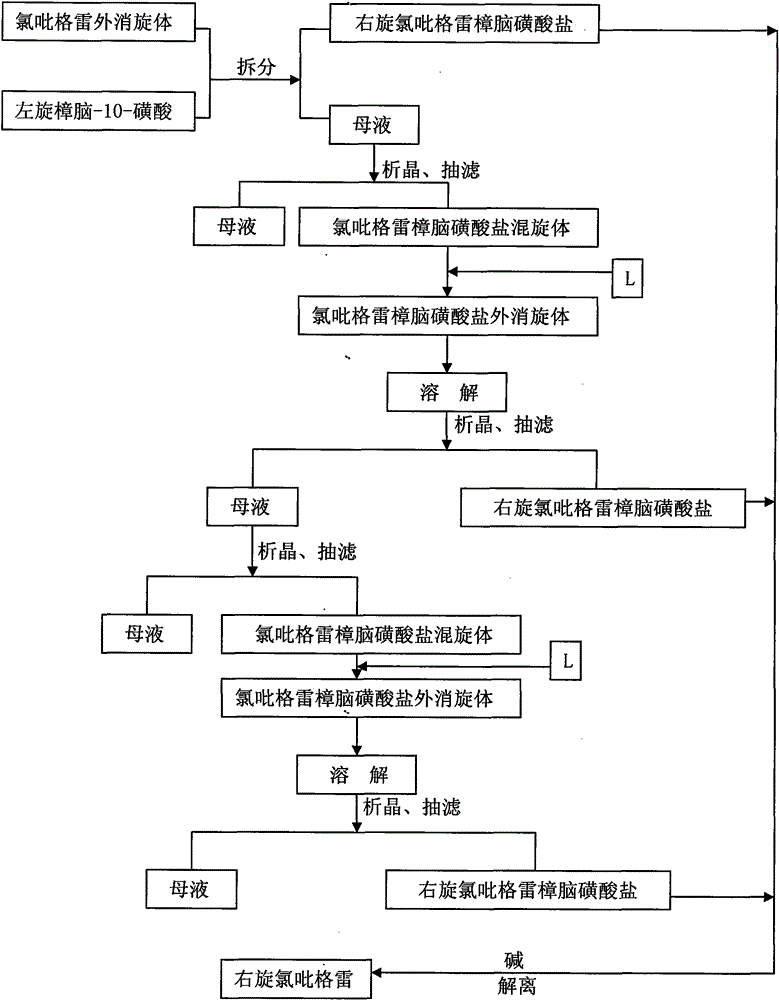
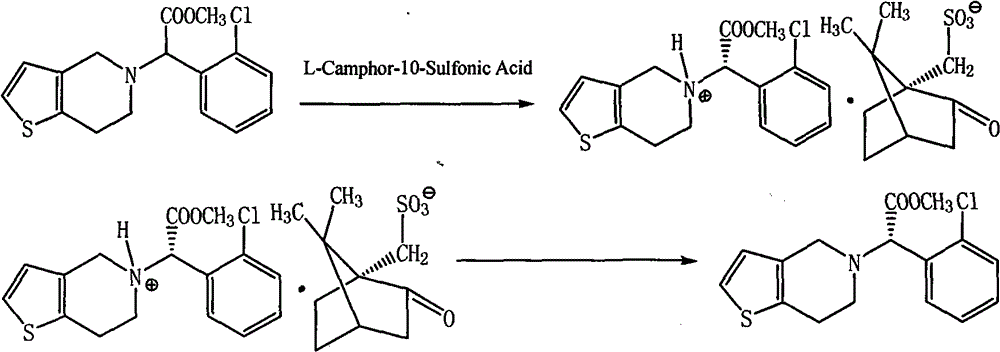
[0024] Step 1: Dissolve 90g (0.28mol) of clopidogrel racemate in 450ml of dichloromethane / acetone mixed solvent (1 / 10 by volume), and add 64.5g (0.28mol) of L-camphor-10-sulfonic acid , stirred and dissolved, stirred and crystallized between 15-20°C for 12 hours, suction filtered (the mother liquor was continued to be used in step 2), and dried to obtain 46.5g of the target product, D-clopidogrel camphorsulfonate B, with an ee value> 99.8%.
[0025] Step 2: Control the temperature of the mother liquor in step 1 to 5-10°C, stir for 4 hours, suction filter and dry to obtain 64g of clopidogrel camphorsulfonate C, and add 4.1g of dextroclopidogrel camphorsulfonate B to C, and add dichloromethane / acetone mixed solvent (volume ratio is 1 / 10) 340ml, heating makes it dissolve, stirring crystallization, stirring crystallization 12 hours between the control temperature 15-20 ℃, then through suction filtration ( The mother liquor was continued to be used in Step 3), and dried to obtain ...
Embodiment 2
[0029] Step 1: Dissolve 180 g (0.56 mol) of clopidogrel racemate in 900 ml of dichloromethane / acetone mixed solvent (1 / 10 by volume), add 129 g (0.56 mol) of L-camphor-10-sulfonic acid, Stir to dissolve, stir and crystallize at 15-20°C for 12 hours, filter with suction (the mother liquor will continue to be used in step 2), and dry to obtain the target product dextroclopidogrel camphorsulfonate solid B is 95g, ee value > 99.8 %.
[0030] Step 2: Control the temperature of the mother liquor in step 1 to 0-5°C, stir for 4 hours, suction filter and dry to obtain 130 g of clopidogrel camphorsulfonate C, and add 8 g of dextroclopidogrel camphorsulfonate B to C , and add 690ml of dichloromethane / acetone mixed solvent (1 / 10 in volume ratio), heat to dissolve, stir and crystallize at 15-20°C for 18 hours, then filter with suction (the mother liquor will continue to be used in step 3), and dry to obtain The solid B' of clopidogrel camphorsulfonate is 44.1 g, and the ee value is >99.8%...
Embodiment 3
[0034] Step 1: Dissolve 180 g (0.56 mol) of clopidogrel racemate in 900 ml of dichloromethane / acetone mixed solvent (1 / 10 by volume), add 129 g (0.56 mol) of L-camphor-10-sulfonic acid, Stir to dissolve, stir and crystallize at 15-20°C for 18 hours, filter with suction (the mother liquor will continue to be used in step 2), and dry to obtain the target product, D-clopidogrel camphorsulfonate B, which is 95.6g, and the ee value is >99.8 %.
[0035]Step 2: Control the temperature of the mother liquor in step 1 to 0-5°C, stir for 8 hours, suction filter, and dry to obtain 141 g of clopidogrel camphorsulfonate C, and add 10 g of dextroclopidogrel camphorsulfonate B to C and add 755ml of dichloromethane / acetone mixed solvent (volume ratio is 1 / 10), heat to dissolve, stir and crystallize, control the temperature between 15-20°C to stir and crystallize for 18 hours, then suction filter (mother liquor Continue to use in step 3), dry to obtain dexclopidogrel camphorsulfonate solid B' ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com