New benzocyclobutane, preparation method thereof and application thereof
A technology for separation and purification of compounds, applied in the preparation of organic compounds, preparation of aminohydroxyl compounds, chemical instruments and methods, etc., can solve problems such as low yield and difficulty in industrialization, and achieve high yield, high optical purity, and resolution Effect of Yield Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
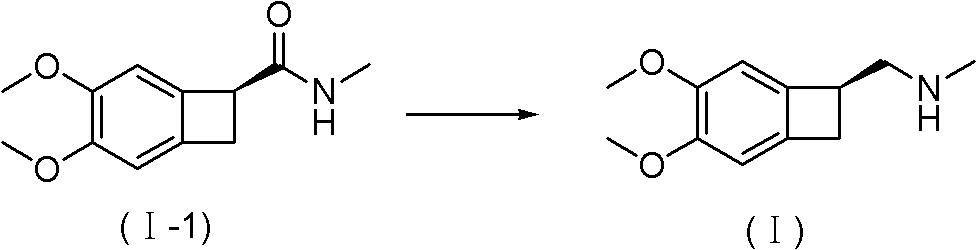
[0048] Embodiment (1S)-4, the synthesis of 5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane oxalate
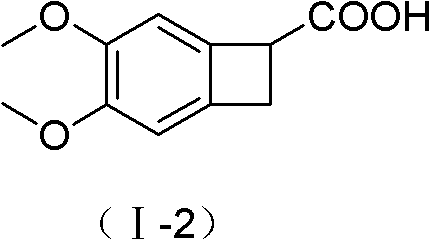
[0049] Step A: Synthesis of I-2 (4,5-dimethoxy-1-carboxy-benzocyclobutane)
[0050] 4,5-dimethoxy-1-cyano-benzocyclobutane (200g, 1.04mol), potassium hydroxide (1000g, 18mol) and ethanol (4000ml) were mixed, stirred and refluxed for 5 hours, and the reaction ended . Cool down to room temperature naturally, add water (10 L) to dissolve, evaporate ethanol under reduced pressure, acidify with concentrated hydrochloric acid, extract with ethyl acetate (1000ml×3), combine the extracts, dry over anhydrous sodium sulfate, decolorize with activated carbon, evaporate the solvent under reduced pressure, Washed with ethyl acetate (600ml) and dried to obtain 208g of the title compound with a yield of 95%. mp137—138°C. TLC: dichloromethane:methanol=10:1, Rf value=0.4.
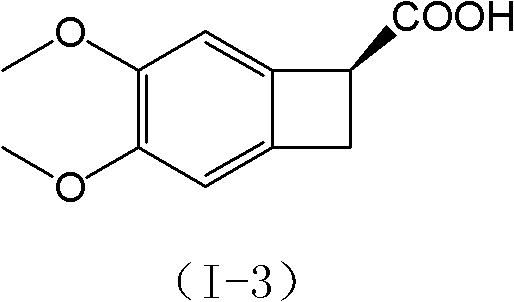
[0051] Step B: Synthesis of I-3 ((1S)-4,5-dimethoxy-1-carboxy-benzocyclobutane)
[0052] Step B-1: Mix the compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com