Photoelectric conversion element and solar cell
A photoelectric conversion element, a general-type technology, applied in the direction of electrical components, photovoltaic power generation, electrolytic capacitors, etc., can solve the problem of low conversion efficiency, and achieve the effect of good adsorption and high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
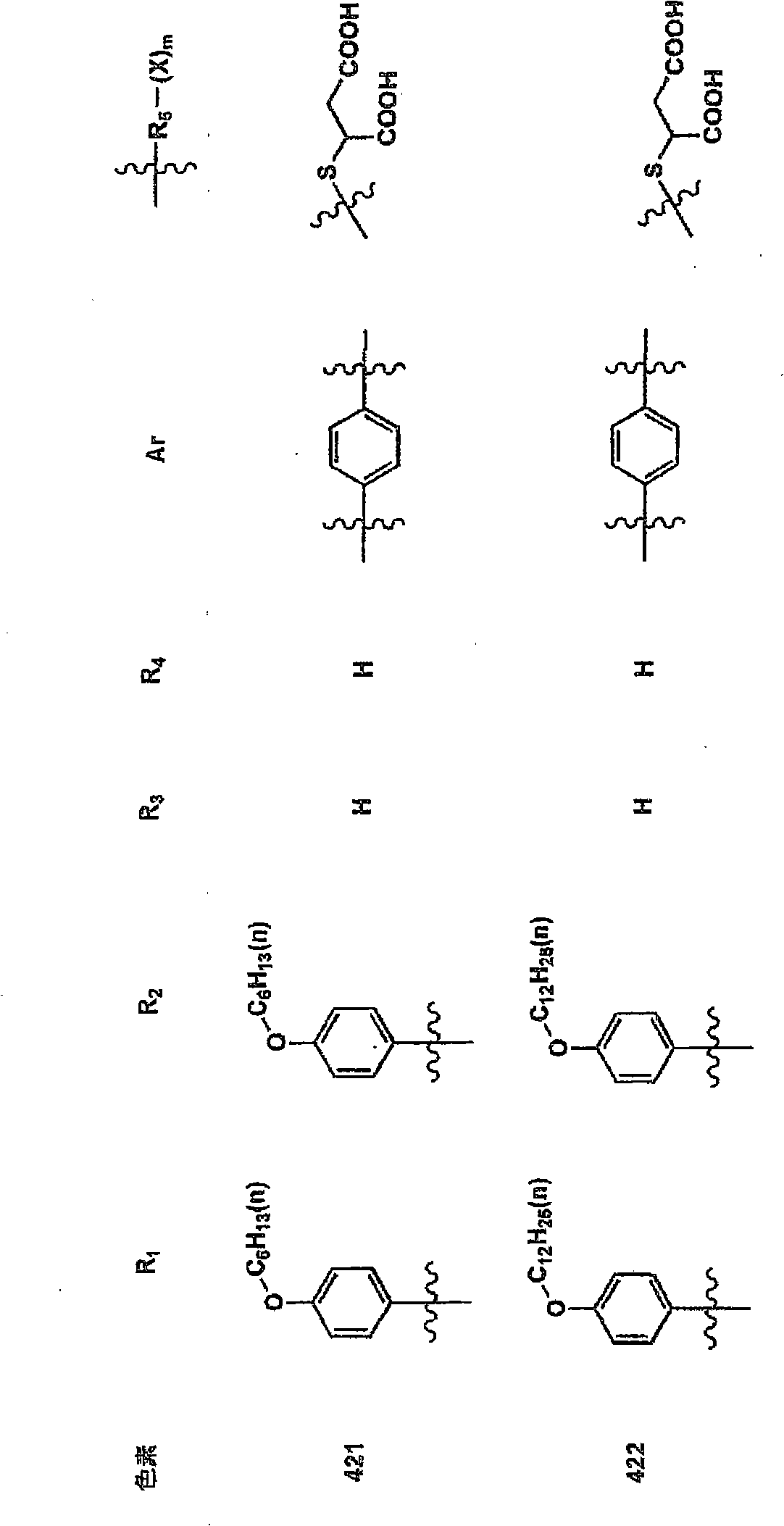
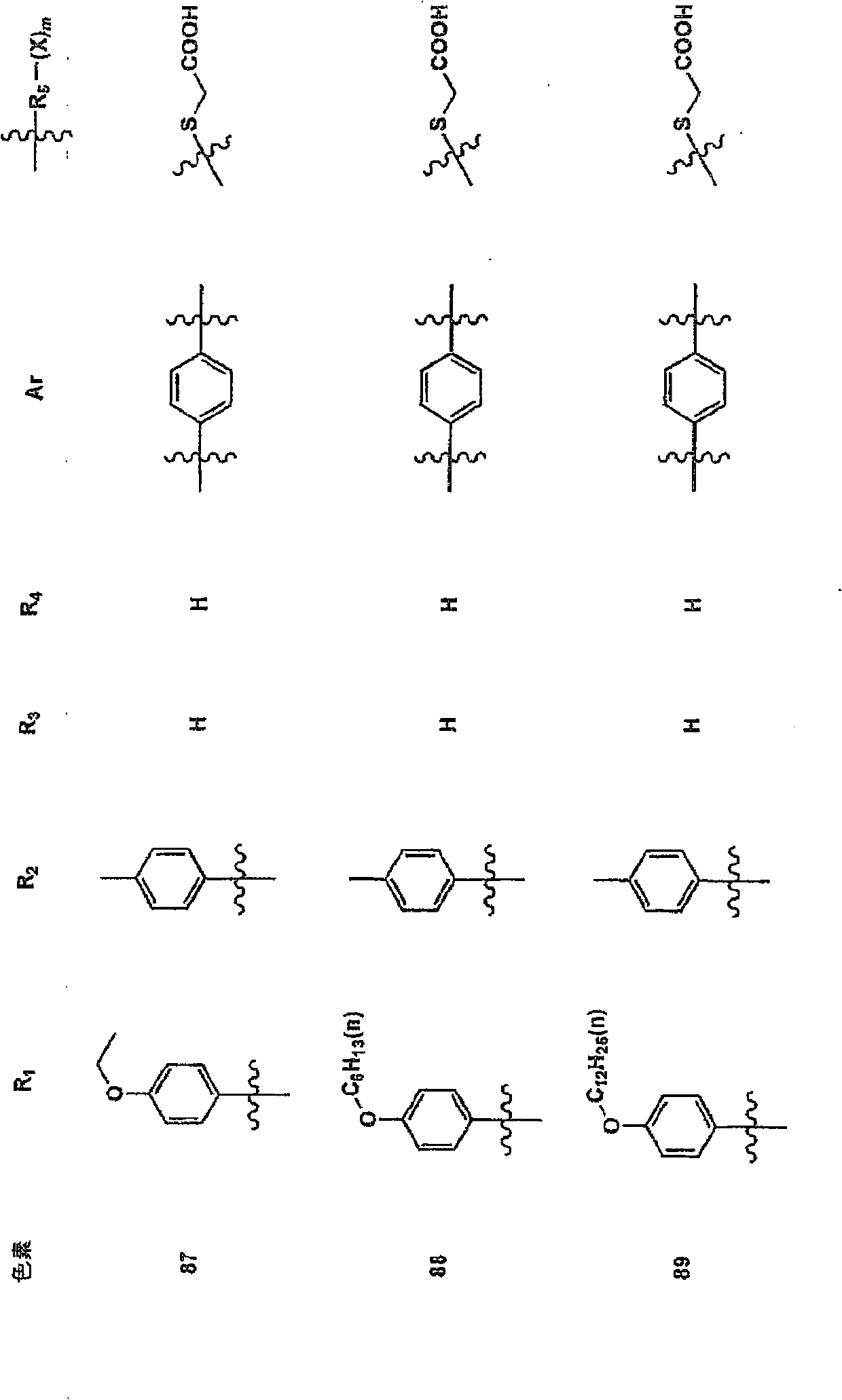
Synthesis Example 1 (Synthesis of Pigment 1)
The aldehyde (compound A) was added to a DMF solution of 2.5 equivalents of diethyl benzhydryl phosphonate and 3 equivalents of K-OtBu, and stirred at 120°C for 1 hour. After adding water to the reaction solution, it was extracted with ethyl acetate, washed with water, dried with magnesium sulfate, concentrated to dryness with a rotary evaporator, and treated with a silica column to obtain compound B.
1.5 equivalents of phosphorus oxychloride and 3 equivalents of DMF were added to the toluene solution of compound B, and stirred at 60°C for 1 hour. Cold water was added to the reaction solution, stirred at room temperature for 1 hour, extracted with ethyl acetate, washed with water, dried with magnesium sulfate, concentrated and dried with a rotary evaporator, and treated with a silica column to obtain compound C .
The acetic acid solution containing compound C, 1.2 equivalents of thiohydantoin, and 3 equivalents of ammonium acetate ...
Example Embodiment
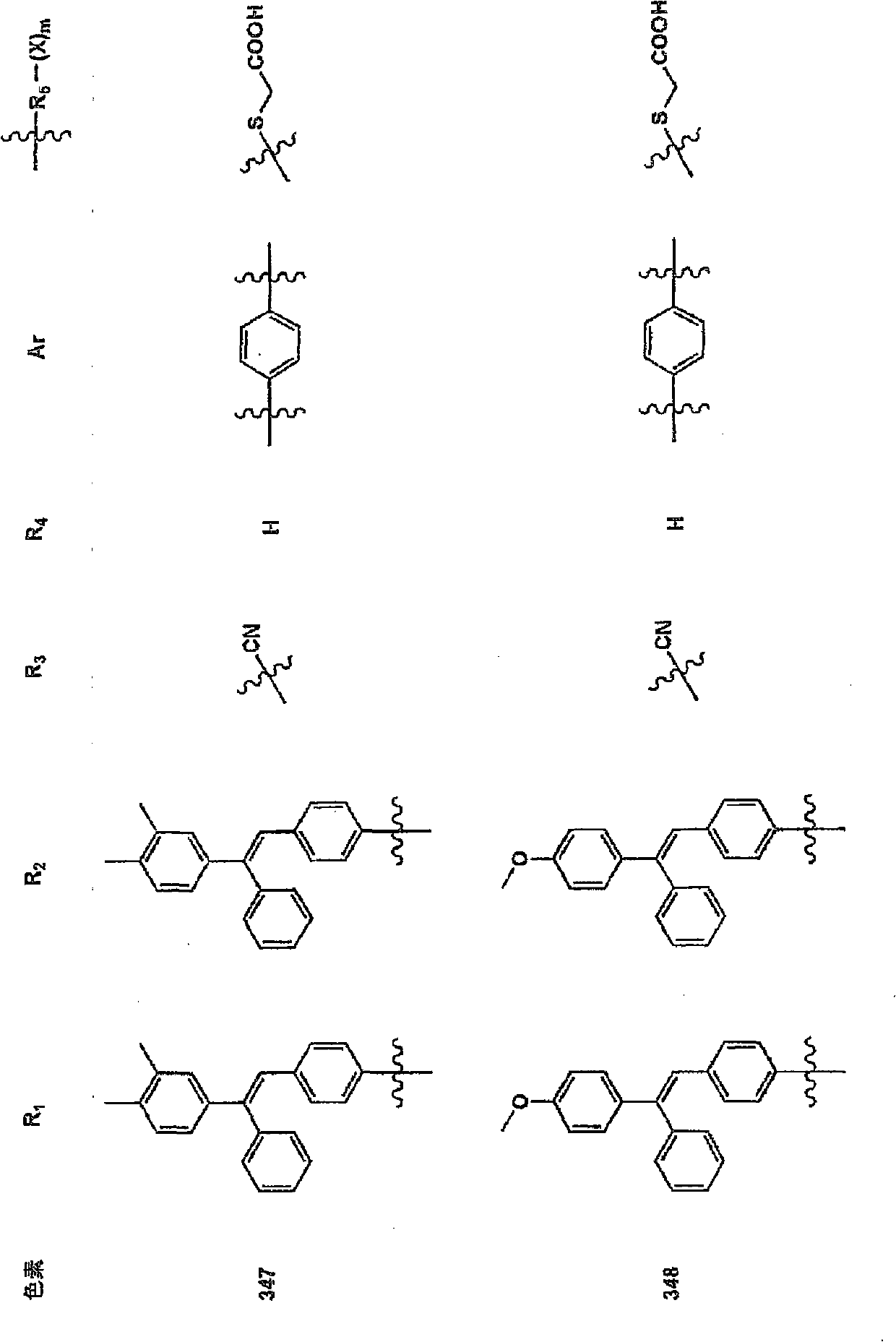
Synthesis Example 2 (Synthesis of Pigment 32)
The dye 32 was synthesized according to the following reaction scheme.
3 equivalents of phosphorus oxychloride was dropped into DMF, and after stirring at room temperature for 30 minutes, DMF of p-methoxytriphenylamine was dropped at 0° C., and the mixture was stirred at room temperature for 3 hours. Cold water was added to the reaction solution, stirred at room temperature for 1 hour, extracted with ethyl acetate, washed with water, dried with magnesium sulfate, concentrated and dried with a rotary evaporator, and treated with a silica column to obtain compound E .
The DMF solution of compound E and 1.05 equivalent of diethylphenyl (p-tolyl) methyl phosphonate was cooled to 0°C, 1.1 equivalent of sodium methoxide was added, and the mixture was stirred for 3 hours. After adding a 0.1 mol / l hydrochloric acid aqueous solution to the reaction solution, it was extracted with ethyl acetate, washed with water, dried with magnesium sulfa...
Example Embodiment
Example
Hereinafter, the present invention will be described through examples, but the present invention is not limited to these examples.
Example
[Production of photoelectric conversion element 1]
Using screen printing method (coating area 5×5mm 2 ) A titanium dioxide paste (anatase type, primary average particle size (average under microscope observation) 18 nm, polyethylene glycol dispersion) was applied to a fluorine-doped tin oxide (FTO) conductive glass substrate. Coating and drying were repeated 3 times (120°C for 3 minutes), firing at 200°C for 10 minutes and 500°C for 15 minutes to obtain a 15 μm thick titanium dioxide thin film. The same method was used to coat and fire titanium dioxide paste (anatase type, primary average particle size (average under microscope) 400nm, polyethylene glycol dispersion) on the film to form a titanium dioxide film with a thickness of 5 μm.
Dissolve the pigment 1 of the present invention in a mixed solvent of acetonitrile: tert-butanol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com