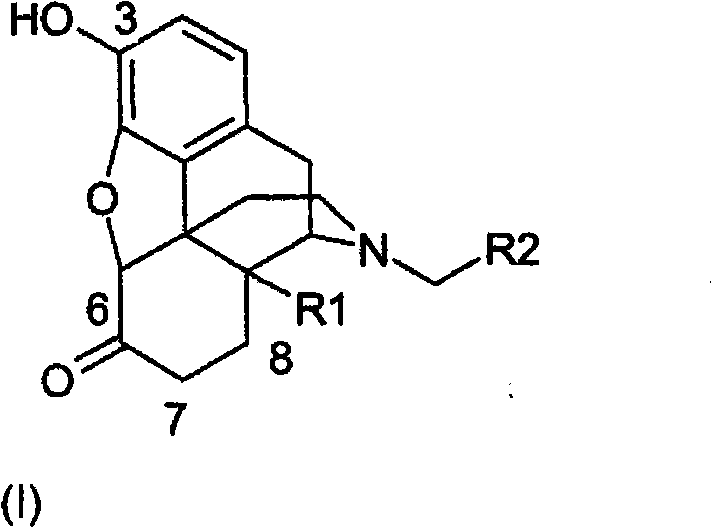
Method for the preparation of morphine compounds
The technology of a compound, morphine, is applied in the field of naloxone, which can solve the problems such as toxicity associated with thiols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
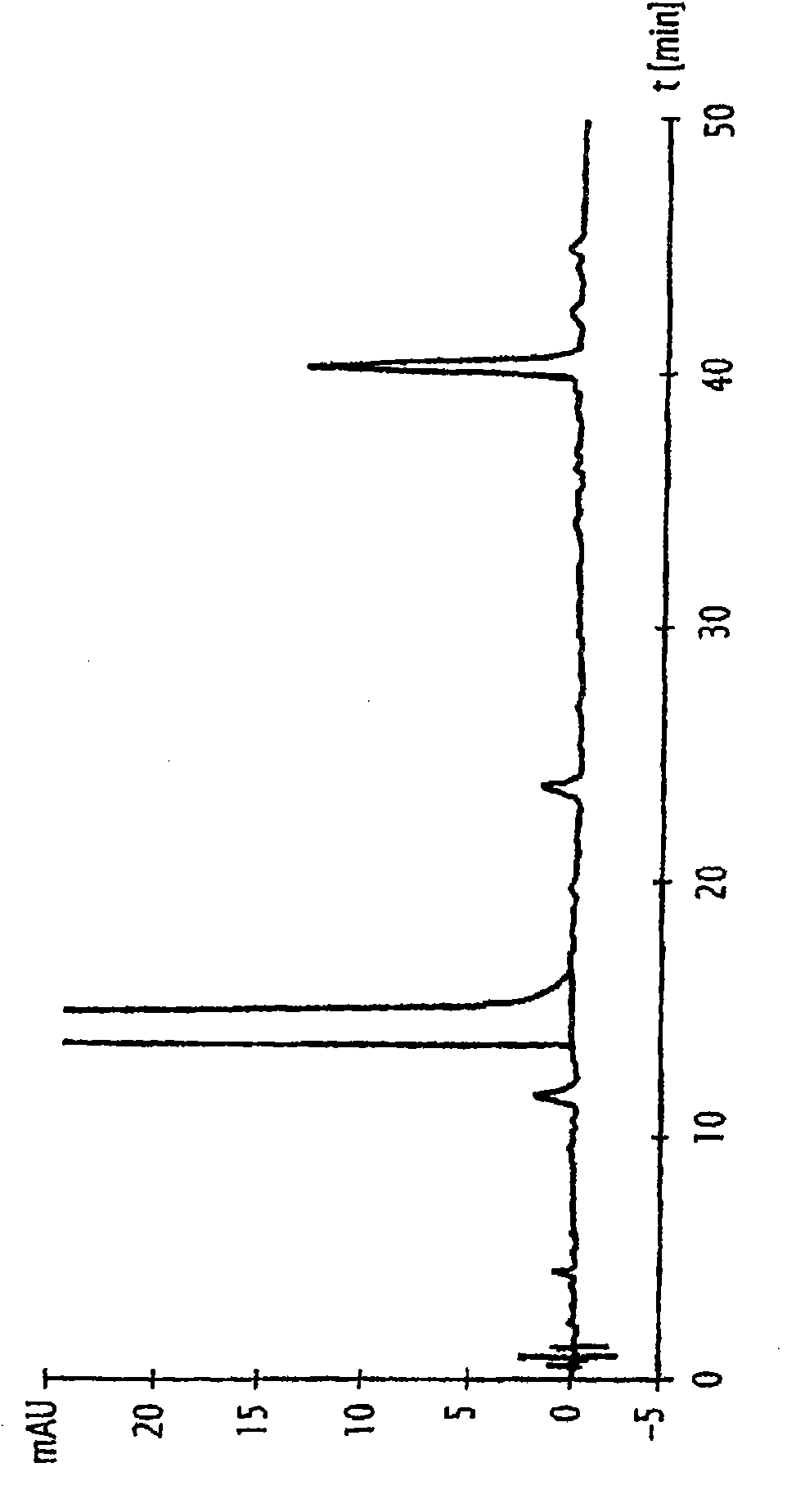
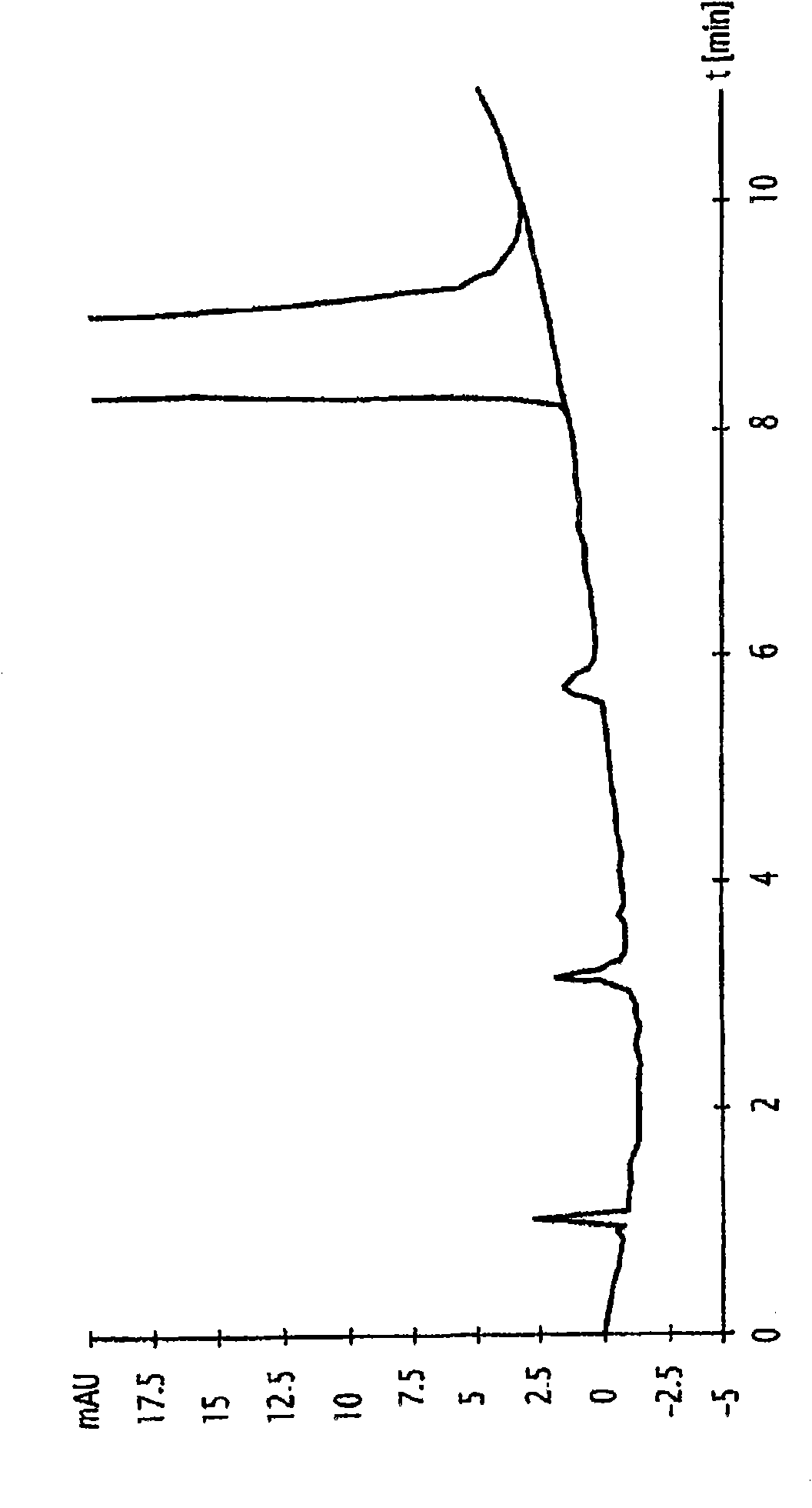
[0127] Example: Preparation of Naloxone Containing Low Content of α,β-Unsaturated Compounds
[0128] 15.08 g (0.046 mol) of crude naloxone base (obtained as follows: in NaHCO 3 In the presence of N-allylation of noroxymorphone hydrochloride with allyl bromide at 60 ° C, and then precipitated from water) into a 250 ml three-necked flask equipped with a thermometer and a magnetic bar, and then added 105ml of water and 9ml (0.06mol, 2eq) of concentrated sodium hydroxide solution (30%). The medium is stirred at ambient temperature until complete dissolution.
[0129] The medium was then added to a dropping funnel and then added dropwise to a three-necked flask containing 36 ml (0.27 mol, 8 equivalents) of concentrated sodium hydroxide solution (30%). The initial ambient temperature of the reaction medium, ie 21.6°C, reached 21.5°C at the end of the addition, and then the pH of the reaction medium was 14.4.
[0130] Stirring was continued for another 30 minutes at this tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com