Method for degrading perfluorinated compound by photo-reduction defluorination
A compound and fluorine-based technology, which is applied in the field of photoreduction defluorination to degrade perfluorinated compounds, can solve the problems of inefficient defluorination of perfluorinated organic compounds, and achieve the effect of reducing product toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
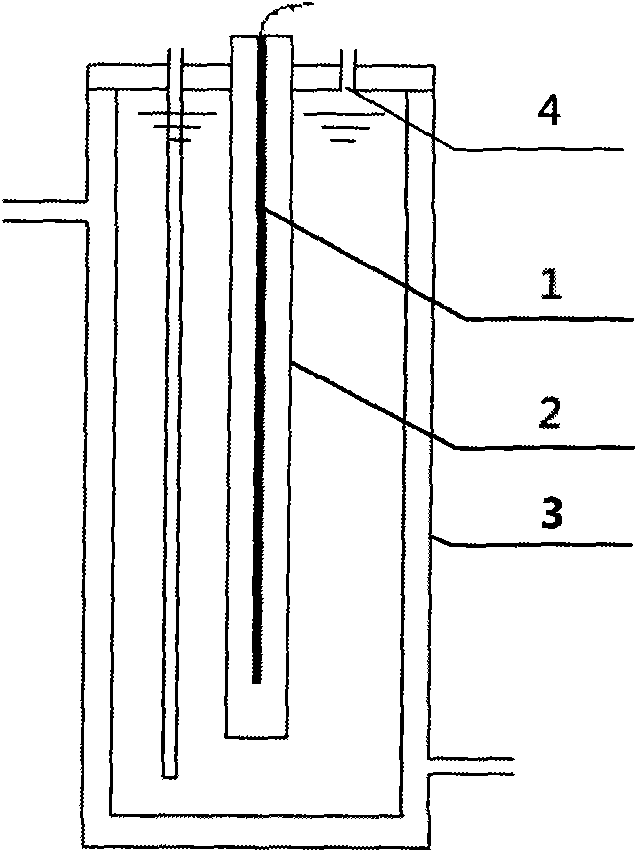
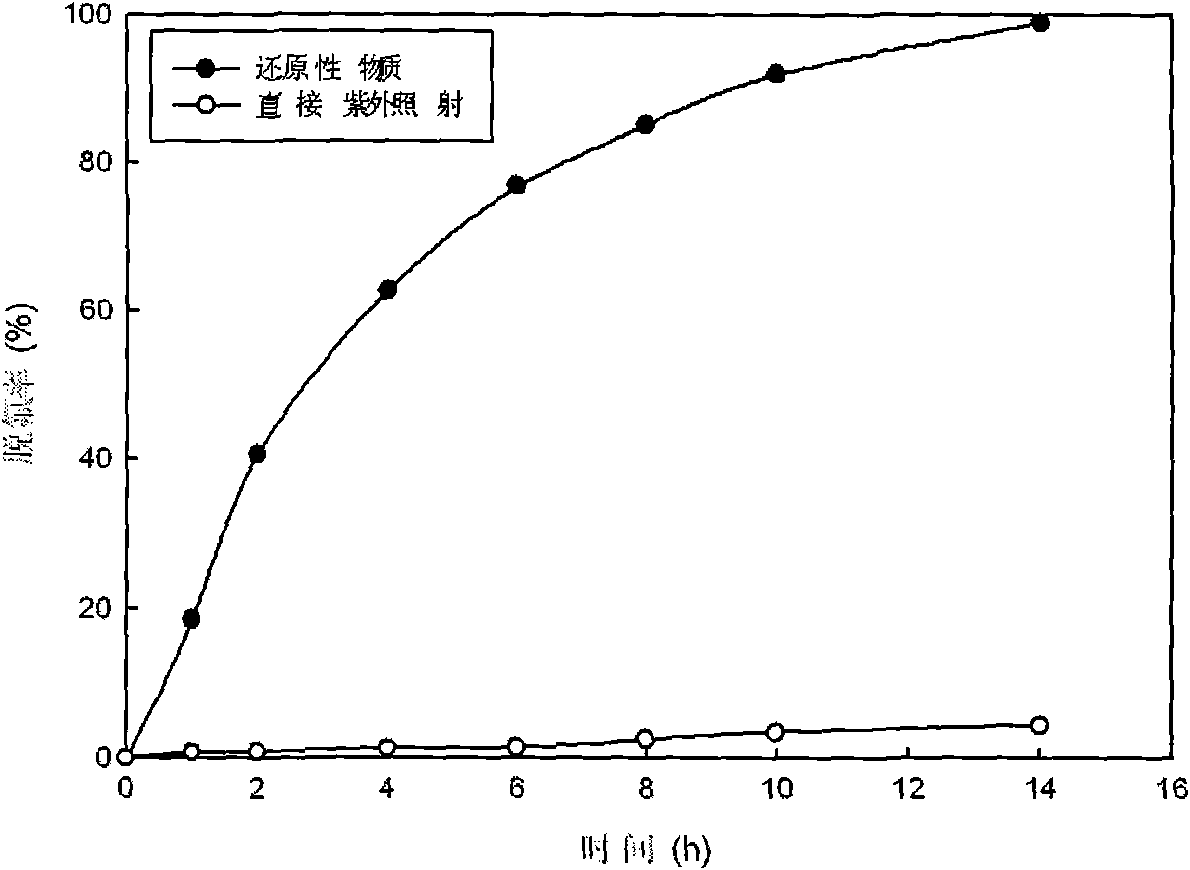
[0029] Such as figure 1 As shown, 720mL containing 0.03mmol / L of perfluorooctanoic acid (PFOA) and 0.6mmol / L of potassium thiosulfate solution is placed in reactor 3, the emission wavelength of UV lamp 1 is adjusted to be 510nm, and the Under the irradiation of UV lamp 1 of 2, PFOA reacts with potassium thiosulfate to start defluorination, and the decomposition effect of defluorination is shown in figure 2 . The defluorination rate is directly calculated according to the fluoride ion concentration in the water during the reaction, and the fluoride ion concentration in the water is determined by ion chromatography. The case of adding reducing substances ( figure 2 marked as "reducing substances"), the fluoride ion concentration in the reaction solution increases with the reaction time, and the defluorination rate of PFOA reaches more than 80% after 8 hours of reaction, and the average PFOA molecule contains 15 fluorine (F) Twelve fluorines had fallen into solution.
Embodiment 2
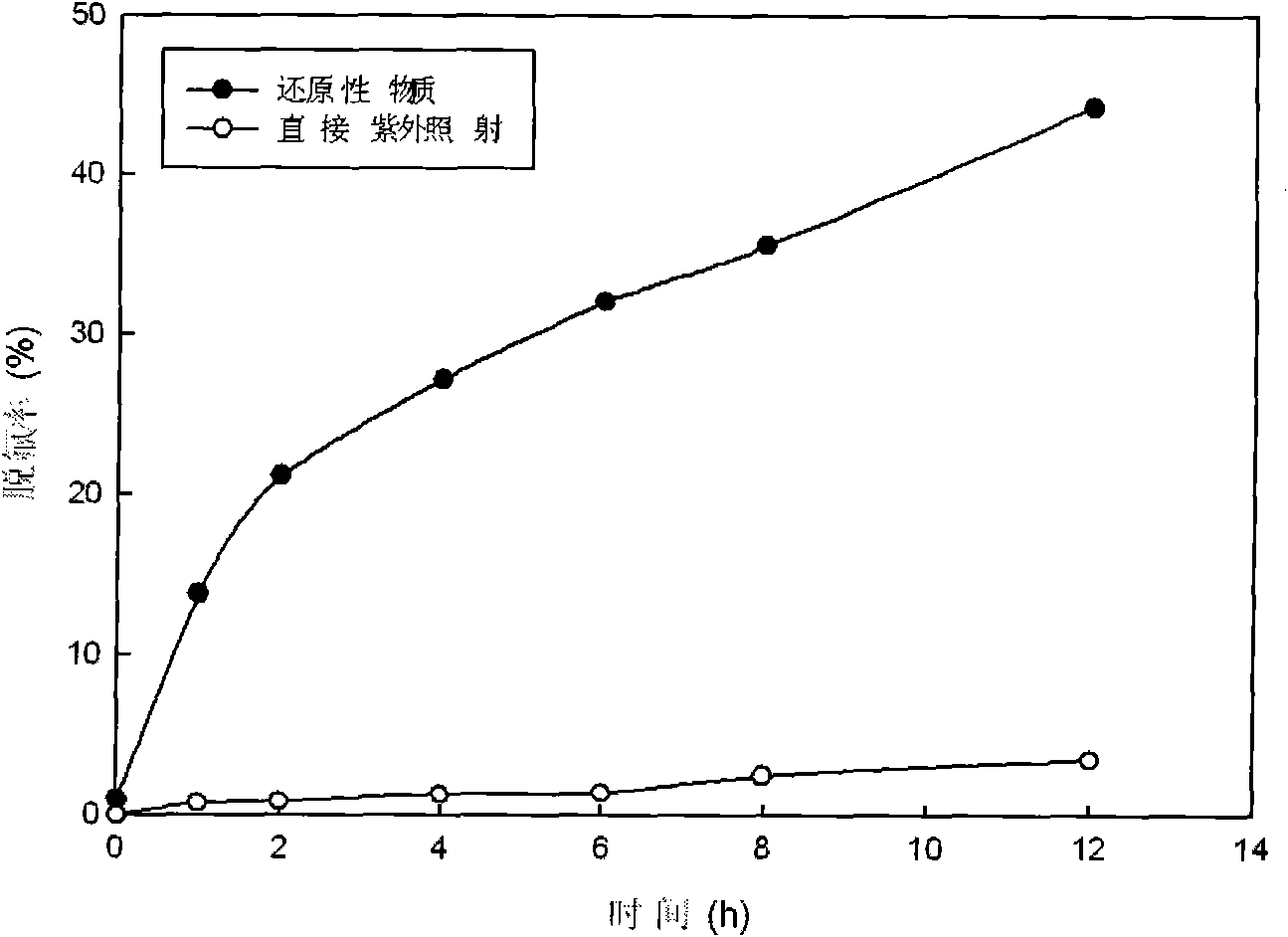
[0031] 720mL containing 0.03mmol / L perfluorooctane sulfonic acid (PFOS) and potassium sulfite solution of 0.09mmol / L are placed in the reactor, and the emission wavelength of the ultraviolet lamp is adjusted to be 475nm. Under the irradiation of a 14W ultraviolet lamp, PFOS React with potassium sulfite to start defluorination, the defluorination decomposition effect can be seen image 3 . The defluorination rate is directly calculated according to the fluoride ion concentration in the water during the reaction, and the fluoride ion concentration in the water is determined by ion chromatography. The case of adding reducing substances ( figure 2 marked as "reducing substances"), the fluoride ion concentration in the reaction solution increases with the reaction time, and the defluorination rate of PFOS reaches more than 35.6% after 8 hours of reaction, and the average PFOS molecule contains 17 fluorine (F) 6.1 pieces of fluorine have fallen into solution.
Embodiment 3
[0033] Place 720mL of perfluorooctane sulfonic acid (PFOS) containing 0.03mmol / L and 0.15mmol / L potassium ferrocyanide solution in the reactor, adjust the emission wavelength of the ultraviolet lamp to 505nm, under the irradiation of a 14W ultraviolet lamp , PFOS reacts with potassium ferrocyanide to start defluorination. The defluorination rate is directly calculated according to the fluoride ion concentration in the water during the reaction, and the fluoride ion concentration in the water is determined by ion chromatography. The concentration of fluorine ions in the reaction solution increases with the reaction time. After 8 hours of reaction, the defluorination rate of PFOS reaches more than 30.4%, and 5.2 of the 17 fluorines (F) contained in the average PFOS molecule have dropped into the solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com