Method for catalytic preparation of amide and derivatives thereof in aqueous phase
A technology for derivatives and compound amides, applied in the field of preparing amides and derivatives thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
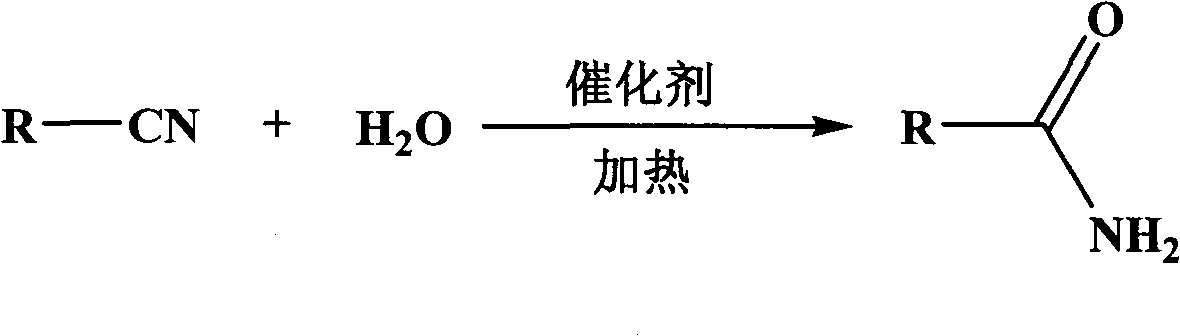
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of benzamide
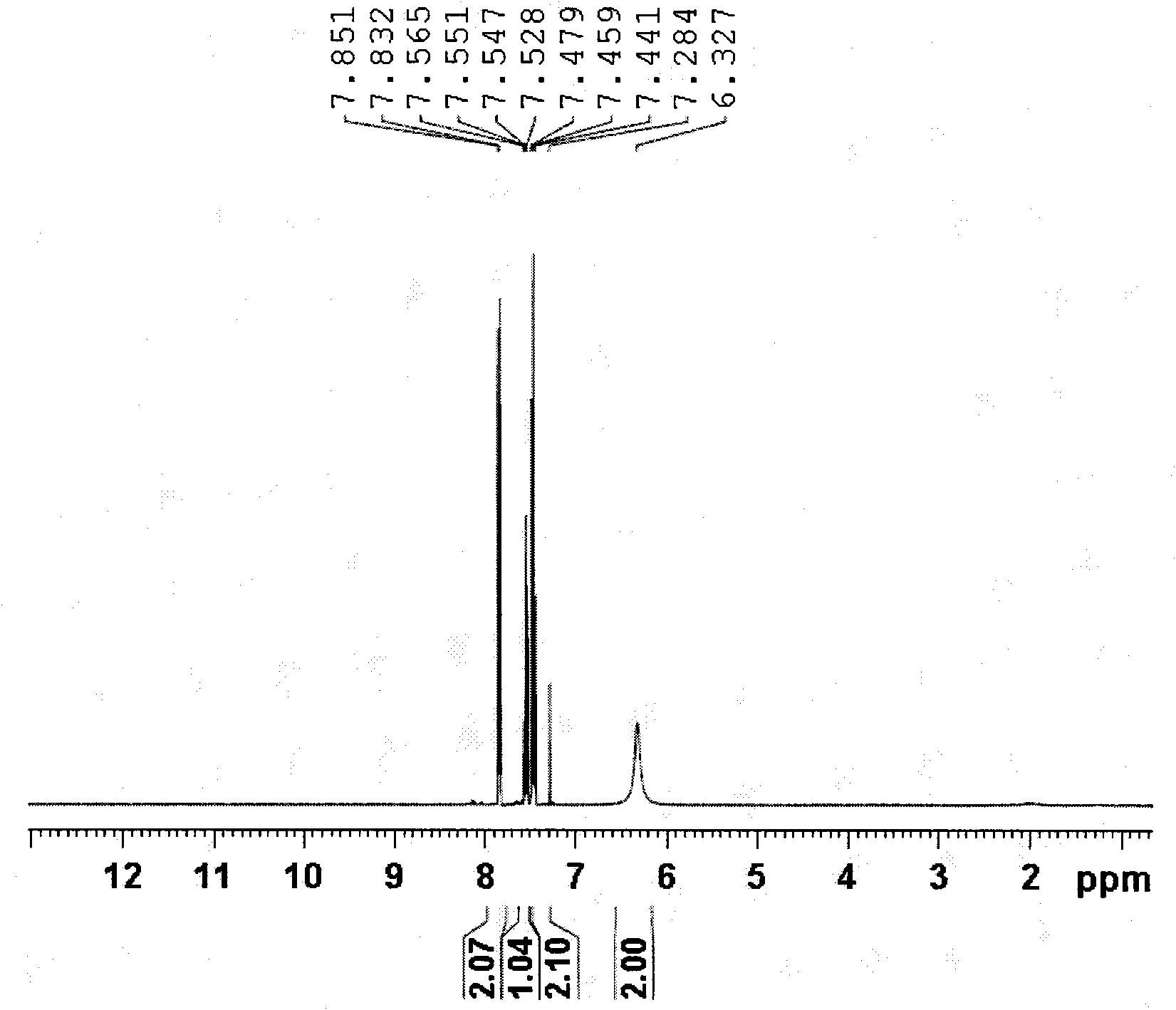
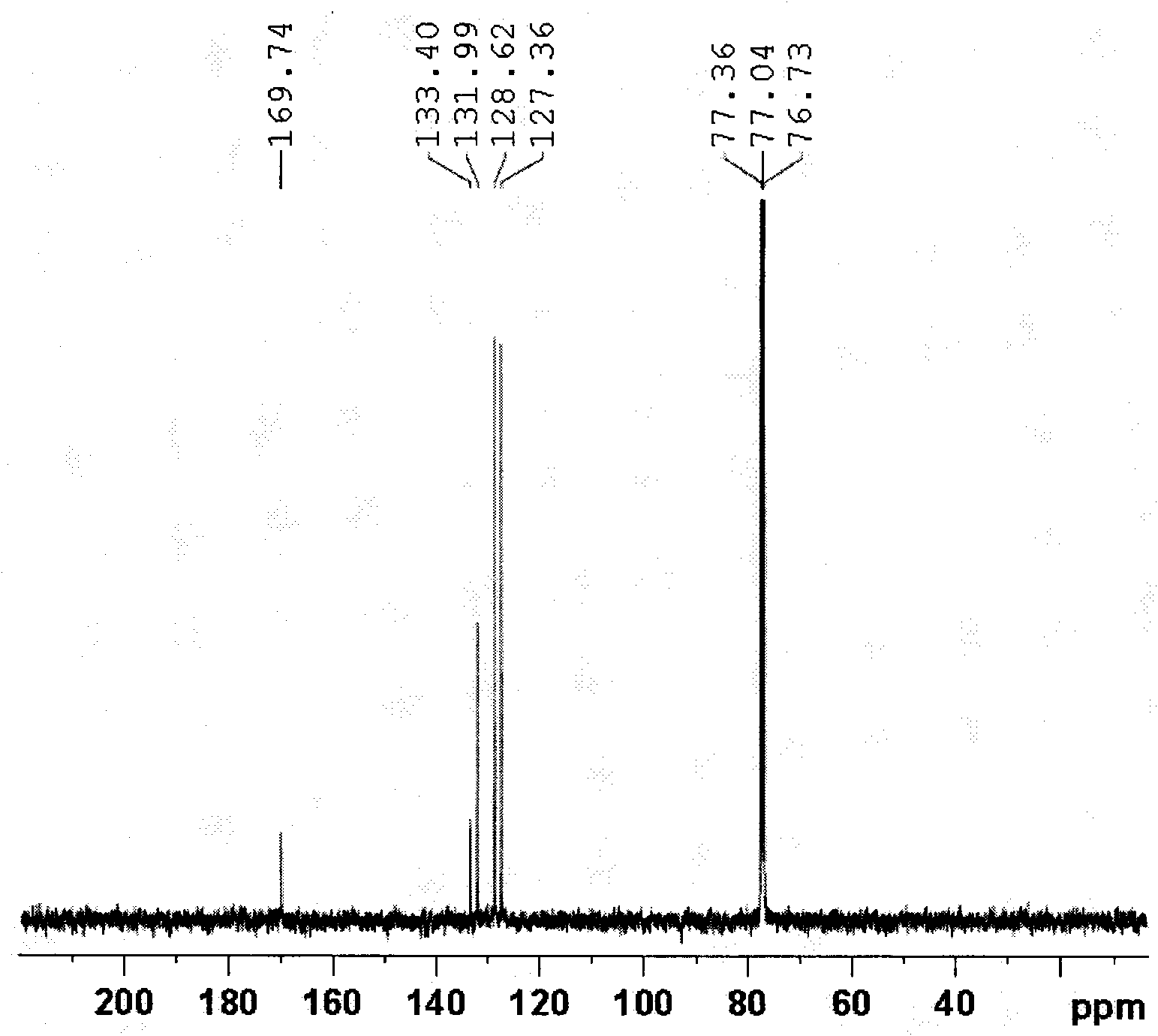
[0024] In the reaction vessel, add cuprous iodide 0.05mmol (9.5mg), 25%-28% ammoniacal liquor 6.8mg, water 2mL, stir at room temperature for 15 minutes, then add benzonitrile 0.5mmol (51.6mg), at 100 It was reacted in an oil bath at ℃ for 21 hours, cooled to room temperature, the reaction mixture was extracted with ethyl acetate, the organic layer was concentrated under reduced pressure, and purified by column chromatography to obtain a white solid product with a yield of 97%. 1 H NMR (400MHz, CDCl 3 ): 7.85 (d, 2H, J = 7.2Hz), 7.55 (t, 1H, J = 7.3Hz), 7.46 (m, 2H), 6.33 (br.s, 2H) ppm. (eg figure 1 ) 13 C NMR (100MHz, CDCl 3 ): δ169.7, 133.4, 132.0, 128.6, 127.4ppm. (such as figure 2 ) MS (EI, m / z): 121 [M + ].
Embodiment 2
[0025] Embodiment 2: the preparation of p-nitrobenzamide
[0026] The preparation method was the same as in Example 1, except that 0.5 mmol (74.0 mg) of p-nitrobenzonitrile was added to obtain a light yellow solid product with a yield of 51%. 1 H NMR (400MHz, DMSO-d 6 ): δ8.39-8.23 (m, 3H), 8.09 (d, 2H, J=8.8Hz), 7.75 (br.s, 1H) ppm. 13 C NMR (100MHz, DMSO-d 6 ): δ166.4, 149.3, 140.2, 129.1, 123.7ppm. MS (EI, m / z): 166 [M + ].
Embodiment 3
[0027] Embodiment 3: the preparation of p-bromobenzamide
[0028] The preparation method was the same as in Example 1, except that 0.5 mmol (91.0 mg) of p-bromobenzonitrile was added to obtain a white solid product with a yield of 87%. 1 H NMR (400MHz, DMSO-d 6 ): δ8.01 (br.s, 1H), 7.78 (d, 2H, J=8.5Hz), 7.64 (d, 2H, J=8.5Hz), 7.42 (br.s, 1H) ppm. 13C NMR (100MHz, DMSO-d 6 ): δ166.5, 133.0, 130.8, 129.2, 124.6ppm.MS (EI, m / z): 200[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com