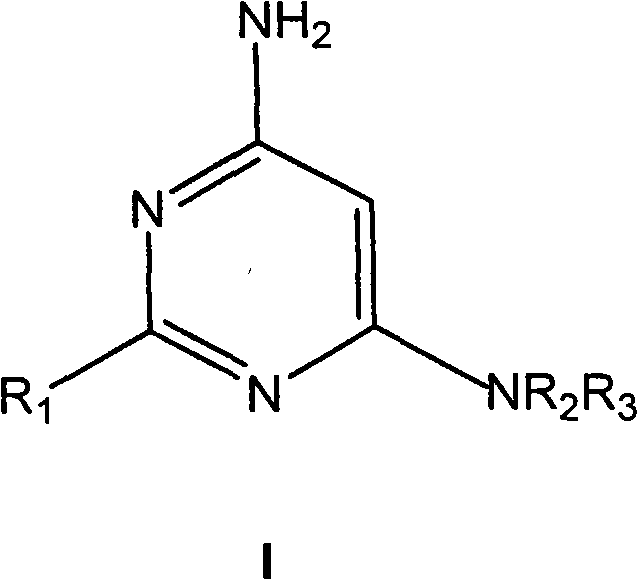
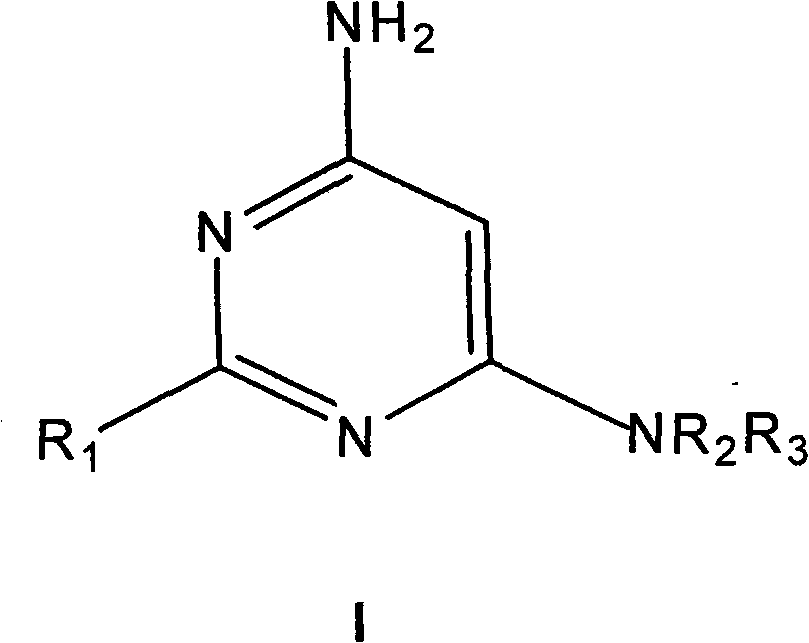
4-aminopyrimidine derivatives as histamine H4 receptor antagonists
An alkyl and group technology, which can be used in anti-inflammatory agents, drug combinations, non-central analgesics, etc., and can solve problems such as low homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0774] 2-isobutyl-6-((3R)-3-(methylamino)pyrrolidin-1-yl)pyrimidin-4-amine
[0775] The compound obtained in Reference Example 1 (144 mg, 0.72 mmol) was added to the compound of Reference Example 3 (89 mg, an approximately 50% mixture of two regioisomers, 0.48 mmol equivalent to 0.24 mmol of the desired region Isomer) and DIEA (0.25ml, 1.44mmol) in n-BuOH (6ml) and heated the resulting mixture in a sealed tube at 100°C overnight. Additional compounds of Reference Example 1 (96 mg, 0.48 mmol) and DIEA (0.25 ml) were added and heated at 100°C for another day. The reactant is cooled and the solvent is evaporated to a dry state. By preparative HPLC-MS (column X-Terra PREP MSC185μm (100mm×19mm), rate 20ml / min, eluent: A=AcN, B=NH 4 HCO 3 75mM, gradient: 0 minutes A 25%; 1 minute A 25%; 11 minutes A 90%; 12 minutes A 90%) Purify the resulting crude product and evaporate the product-containing fraction to dryness to form a Boc-protected intermediate Product (26.9 mg, yield 32%). To t...
Embodiment 2 to 3
[0778] Following a method similar to that described in Example 1, but using appropriate starting materials, the following compounds were obtained:
[0779]
Embodiment 4
[0781] 2-isobutyl-6-(3-(methylamino)azetidin-1-yl)pyrimidin-4-amine
[0782] Method A
[0783] The compound of Reference Example 18 (2g, 6.0 mmol), benzophenone imine (1.1ml, 6.60 mmol), sodium tert-butoxide (0.86g, 9.0 mmol), racemic BINAP (0.22g) , 0.36 mmol) and toluene (60ml) were placed in a schlenk tube. The system was cleaned 3 times by vacuum / argon cycle. Then add Pd 2 (dba) 3 (0.11 g, 0.12 mmol) and vacuum / argon purge again 3 times. The reaction mixture was heated overnight at 85°C. Cool the crude reaction product at room temperature and pass through diatomaceous earth Filter and rinse the filter cake with ethyl acetate. The filtrate was evaporated to a dry state. The residue was dissolved in THF (127ml), then 3N HCl (127ml) was added and stirred at room temperature for 3 hours. Evaporate to dryness. The crude product was dissolved in water and ethyl acetate, the phases were separated and the organic layer was discarded. Add 0.5N NaOH to the acidic aqueous phase u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com