Application of 3,15-dicarbonyl gibberellic acid methyl ester in reversing tumor multidrug resistance
A technology of methyl dicarbonyl gibberellate and multidrug resistance is applied in the field of newly synthesized methyl gibberellate, which can solve the problems of no drug-resistant tumor anticancer drugs, inability to know the reversal of tumor drug resistance, etc. The effect of improving curative effect, few reaction steps and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
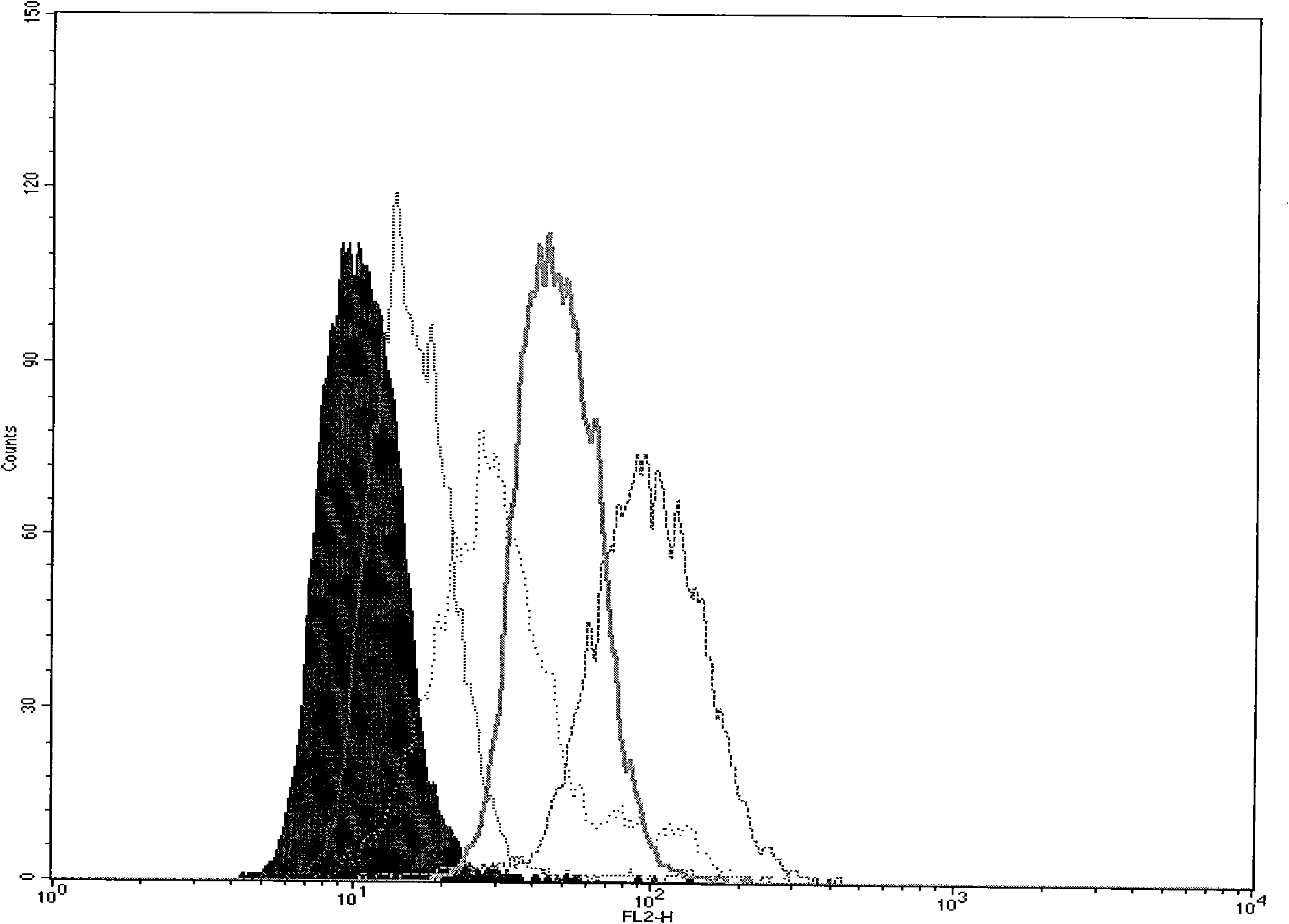
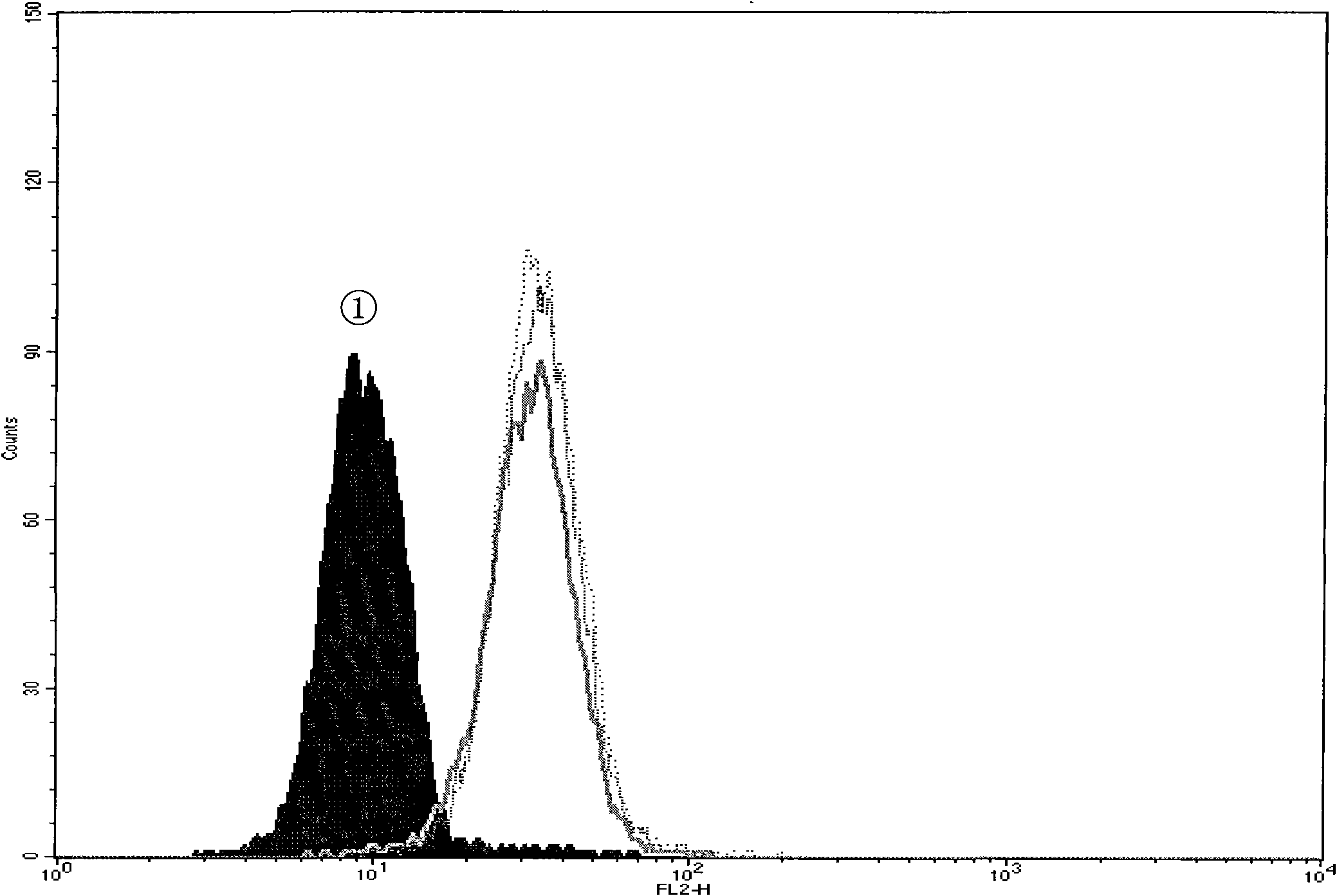
[0102] The multidrug resistance characteristic and degree of embodiment one K562 / A02
[0103] 1. Drugs and preparation
[0104] The chemotherapeutic drugs ADM, EPI, and VP-16 were set at 5 test concentrations of 100, 50, 25, 12.5, and 6.25 μg / ml, which were set according to the results of the preliminary test. The specific preparation is as follows: Weigh a certain amount of medicine and add a certain amount of N.S to dissolve it, then add a certain amount of RPMI1640 complete culture medium to prepare a high-concentration solution, and the remaining concentrations are prepared by diluting in equal proportions.
[0105] 2. Experimental method
[0106] Referring to literature [13], the effect of each compound on cell proliferation was determined by the improved MTT method. Take the K562 and K562 / A02 cell lines in the logarithmic growth phase and adjust the cell suspension to a certain concentration and inoculate them in a 96-well culture plate, 90 μl / well. Immediately after s...
Embodiment 2 56
[0116] Example 2 Sensitivity of K562 / A02 cells to compound GA
[0117] 1. Drugs and preparation
[0118] ADM, VCR, VP-16, and GA are all set at 5 test concentrations of 100, 50, 25, 12.5, and 6.25 μg / ml (this concentration is set according to the results of the preliminary test), and the specific preparation method is the same as before.
[0119] 2. Experimental method
[0120] The improved MTT method is the same as before.
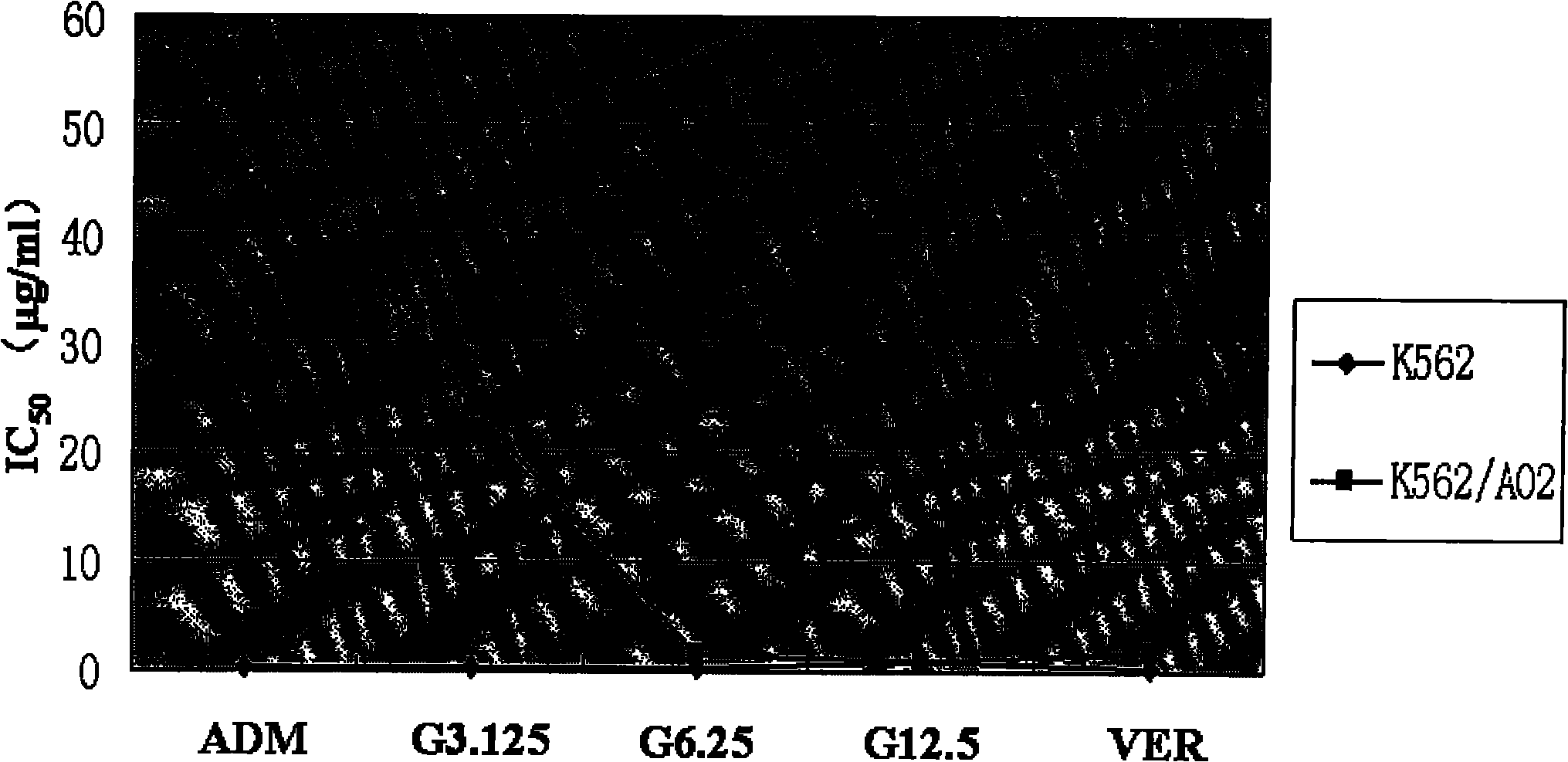
[0121] Results: Compound GA showed higher drug sensitivity when acting on K562 / A02 cells, IC 50 The value is only 4.43 μg / ml (Table 2), but for the other three chemotherapeutic drugs with different structures and mechanisms of action, K562 / A02 cells are not sensitive to their drug effects. It suggested that while K562 / A02 cells were resistant to a variety of chemotherapeutic drugs, they still had high sensitivity to the compound GA, which provided a basis for subsequent experiments.
[0122] Table 2 K562 / A02 cell drug sensitivity to each test substanc...
Embodiment 3
[0129] Example 3 Compound GA reverses the effect and intensity of ADM drug resistance in K562 / A02 cells
[0130] 1. Drugs and preparation
[0131] (1) Compound G
[0132] Set 12.5, 6.25, 3.125 μg / ml 3 test concentrations (this concentration is set according to the results of the preliminary test). The specific preparation is as follows: Weigh a certain amount of GA, add a certain amount of DMSO and wait for it to dissolve, then add a certain amount of RPMI1640 complete culture medium to prepare a high-concentration solution, and each low-concentration solution is prepared by diluting in proportion;
[0133] (2) Positive control VER
[0134] VER is a liquid preparation for injection with an initial concentration of 2.5 mg / ml, adding a certain amount of RPMI1640 complete culture solution to dilute it to a working solution with a concentration of 10 μg / ml;
[0135] (3)ADM
[0136] The stock solution concentration is 1 mg / ml, and a certain amount of RPMI1640 complete culture s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com