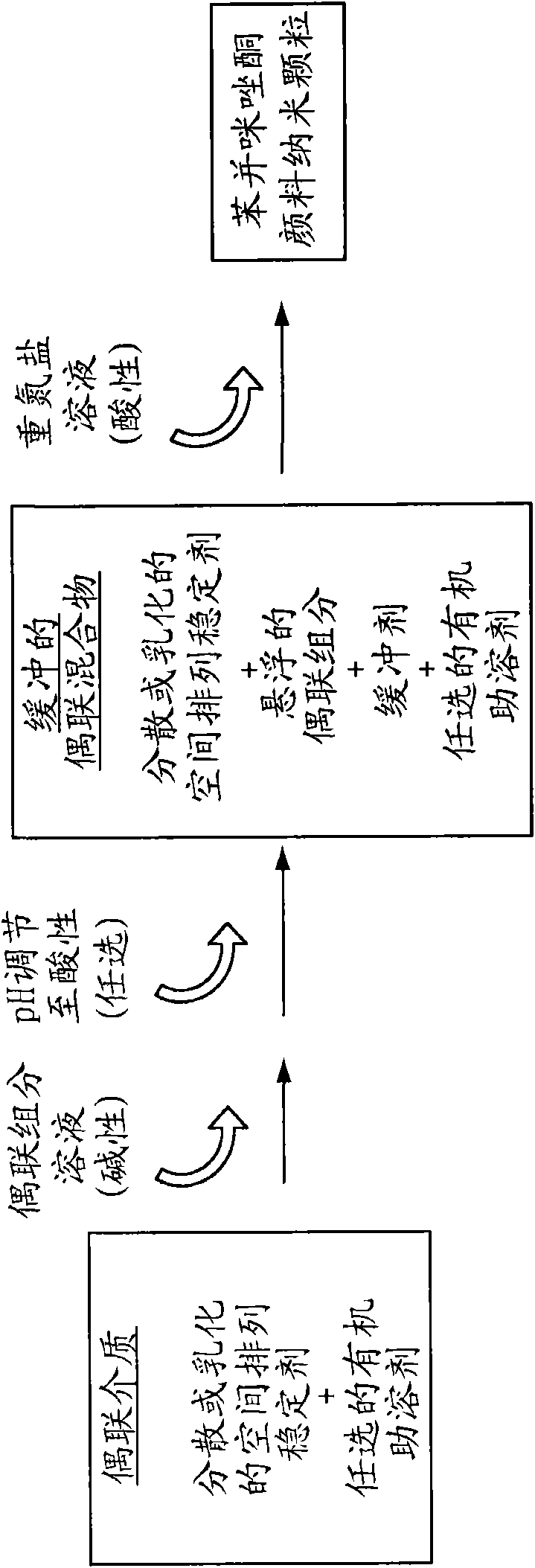
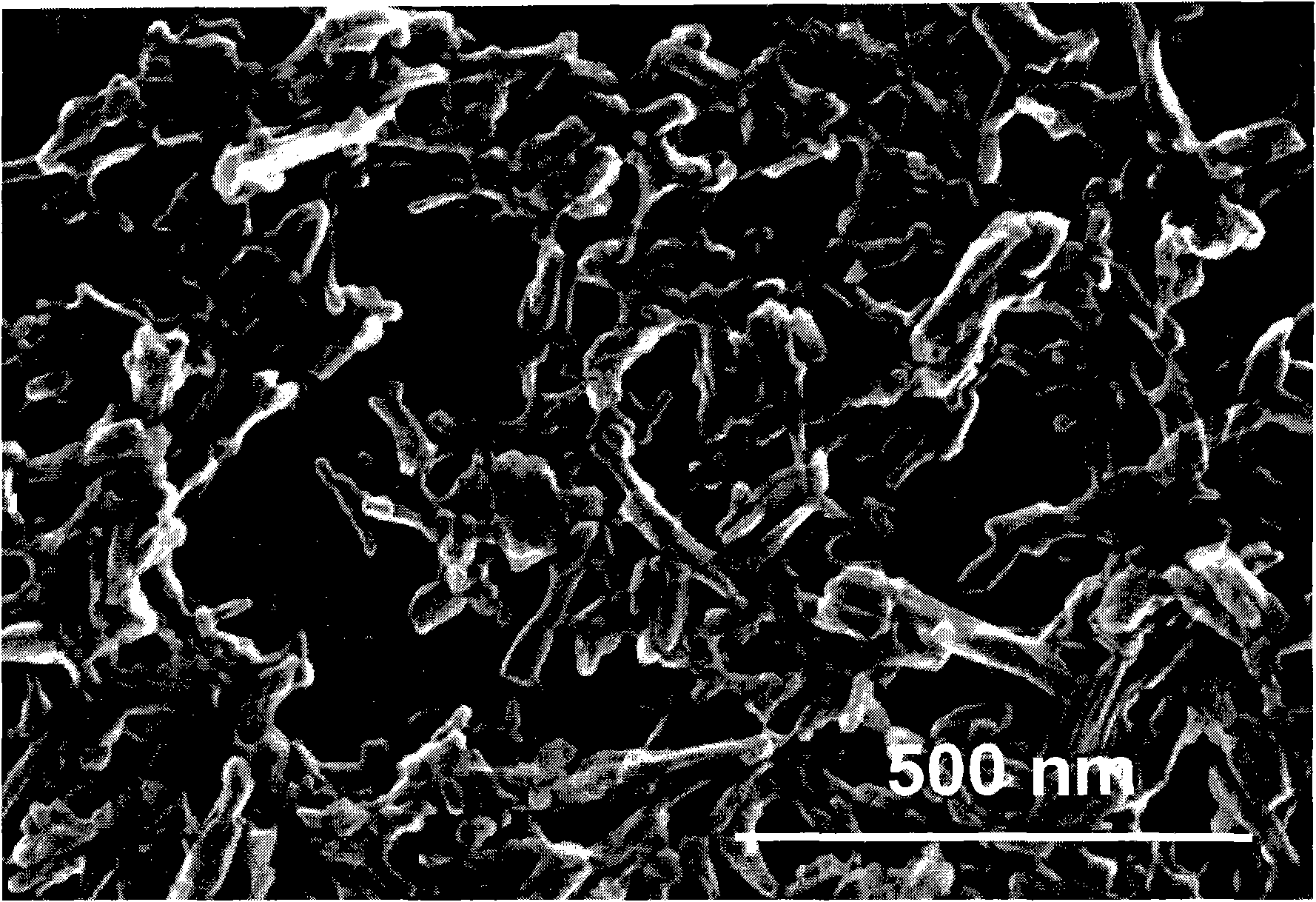
Nanosized particles of benzimidazolone pigments
A technology of benzimidazolone and pigment particles, which is applied in the direction of pigment slurry, nanotechnology, nanotechnology, etc., and can solve problems such as safe ink jetting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1 : Synthesis of Alkylated Benzoic Acid Steric Stabilizer (entry #10 of Table 1)
[0132]
[0133] In a 100 mL vessel, 1.15 g (3.13 mmol) of 2-decyltetradecanoic acid (purchased as ISOCARB 24 from Sasol America) was dissolved in 20 mL of THF under an inert atmosphere with stirring. The solution was cooled to 0°C, and 1.1 mL (12.6 mmol) of oxalyl chloride was slowly added dropwise. Then 4 drops of DMF were added and HCl started to precipitate. The reaction was then slowly warmed to room temperature. After gas evolution had ceased, the reaction was stirred for an additional 30 minutes before the solvent was removed by rotary evaporation. The crude acid chloride was then dissolved in 10 mL of dry THF and stored under an inert atmosphere for a short time.
[0134] To a second 100 mL vessel was added 260.8 mg (1.9 mmol) of methyl 3,5-diaminobenzoate dissolved in 5 mL of dry THF under an inert atmosphere. Triethylamine (0.7 mL, 4.99 mmol) was then added and t...
Embodiment 2
[0136] Example 2 : Synthesis of 5-(2'-decyltetradecanoyl)isophthalic acid steric stabilizer (Table 1, entry #53).
[0137]
[0138] In a 500 mL vessel, 7.65 g (20.8 mmol) of 2-decyltetradecanoic acid (ISOCARB 24, Sasol) suspended in 100 mL of THF was added with stirring under an inert atmosphere. The solution was then cooled to 0 °C, after which 3.5 mL (42 mmol) of oxalyl chloride was slowly added dropwise. Then 0.28 mL of DMF (3.62 mmol) was added and HCl gas began to evolve. After 10 minutes, gas evolution had ceased and the reaction was allowed to warm slowly to room temperature and stir for 3 hours to give a clear colorless solution. The solvent was then removed by rotary evaporation to give a light yellow syrup.
[0139] In a 250 mL vessel, 4.40 g of dimethyl 5-aminoisophthalate (Aldrich, 21.0 mmol) was suspended in 100 mL of dry THF under an inert atmosphere with magnetic stirring. The dimethyl 5-aminoisophthalate suspension was cooled to 0° C., and an ice-cooled...
Embodiment 3
[0141] Example 3 : Synthesis of Pigment Yellow 151 Nanoparticles Using a Steric Stabilizer
[0142] Step I: Diazotization
[0143]In a 100 mL vessel equipped with a thermometer, mix 1.86 g of anthranilic acid (13.6 mmol), 25 mL of deionized water, and 6.5 mL of 5M hydrochloric acid with a magnetic stirrer. The clear solution was cooled to 0 °C, after which 1 mL of ice-cold 6.0 M NaNO was added at a rate to maintain the internal temperature at 0 °C 2 (14.9 mmol) in water. The solution was then stirred at 0°C for at least 30 minutes.
[0144] Step II: Preparation of Coupling Component Mixture
[0145] Add 100 mL of deionized water to a 500 mL container. With vigorous stirring, 0.500 g of 3,5-bis(2'-decyltetradecylamidobenzoic acid) (10 wt % theoretical pigment yield; 0.585 mmol) prepared according to Example 1 was then slowly added dropwise in 12.5 mL solution in isopropanol. 5-Acetoacetamido-benzimidazolone (3.17 g, 13.6 mmol, TCI America) was then added, followed by sl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com