Methods and compositions for treating respiratory disease
A composition and respiratory technology, applied in the field of treatment of respiratory diseases and composition, can solve problems such as side effects and unsafe treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Lung Exposure and Particle Size Analysis
[0056] 6,7-Bis(3-hydroxyphenyl)-pteridine-2,4-diamine (compound A) was formulated as a 20% suspension in 5% tyloxapol in water. use a colloid mill MK module, at 12,000 rpm for 6 hours while keeping the temperature below 50°C), resulting in particle size reduction. Samples of Compound A at 4%, 0.4%, 0.04%, and 0.004% were prepared by dilution with deionized water.
[0057] Nebulization of the suspension was performed using a Pari LC Plus @ 3 LPM with an active diluter at 5 LPM in a nose-only exposure chamber from CH technologies. Aerodynamic particle size was determined by a 7 stage impactor from Intox at 1 LPM. Stages were extracted with acetonitrile:water (1:1) containing 0.05% TFA and analyzed by HPCL against an external standard.
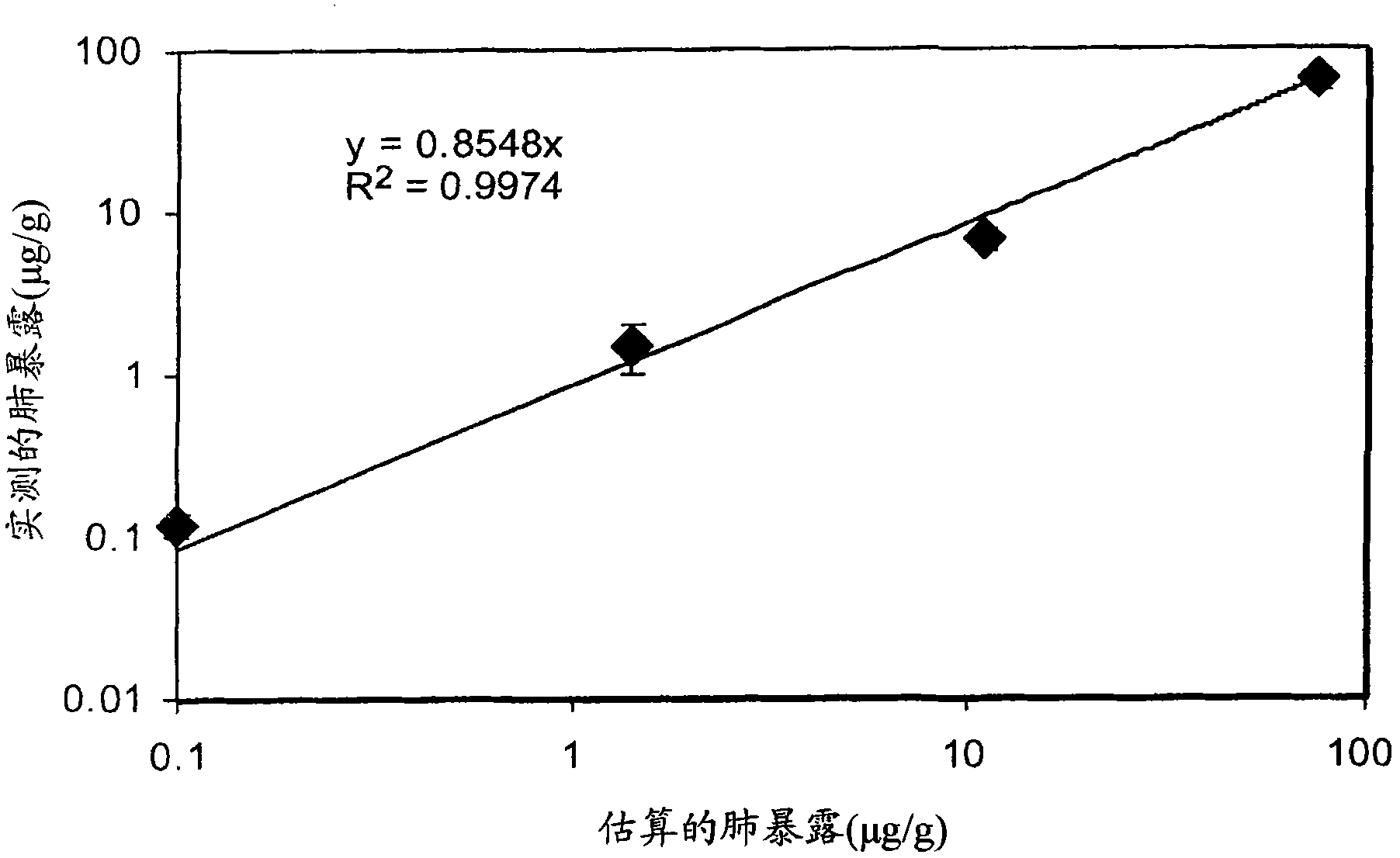
[0058] Estimated lung exposure was calculated based on the following assumptions: absorbable dose collected by a 7-stage impactor; 30 min exposure time; mouse minute volume (mouse m...
Embodiment 2
[0063] Example 2 Inhalation exposure (Inhalation exposure)
[0064] A series of compositions for pulmonary delivery were prepared by preparing a 20% suspension of Compound A in 5% tyloxapol in water. use a colloid mill MK module, at 12,000 rpm for 6 hours while keeping the temperature below 50°C), resulting in particle size reduction. A 4% w / v sample of compound A was prepared by dilution with deionized water.
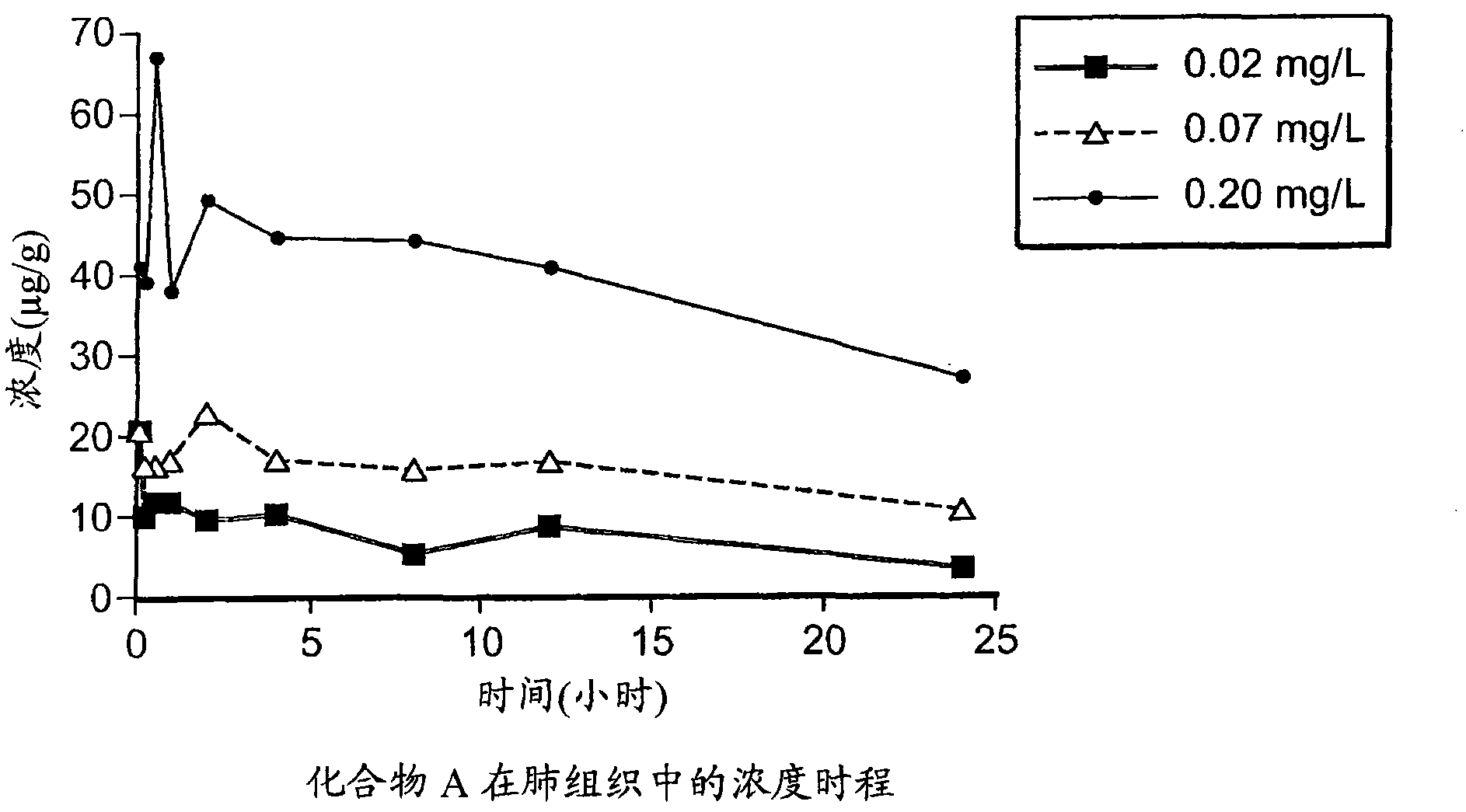
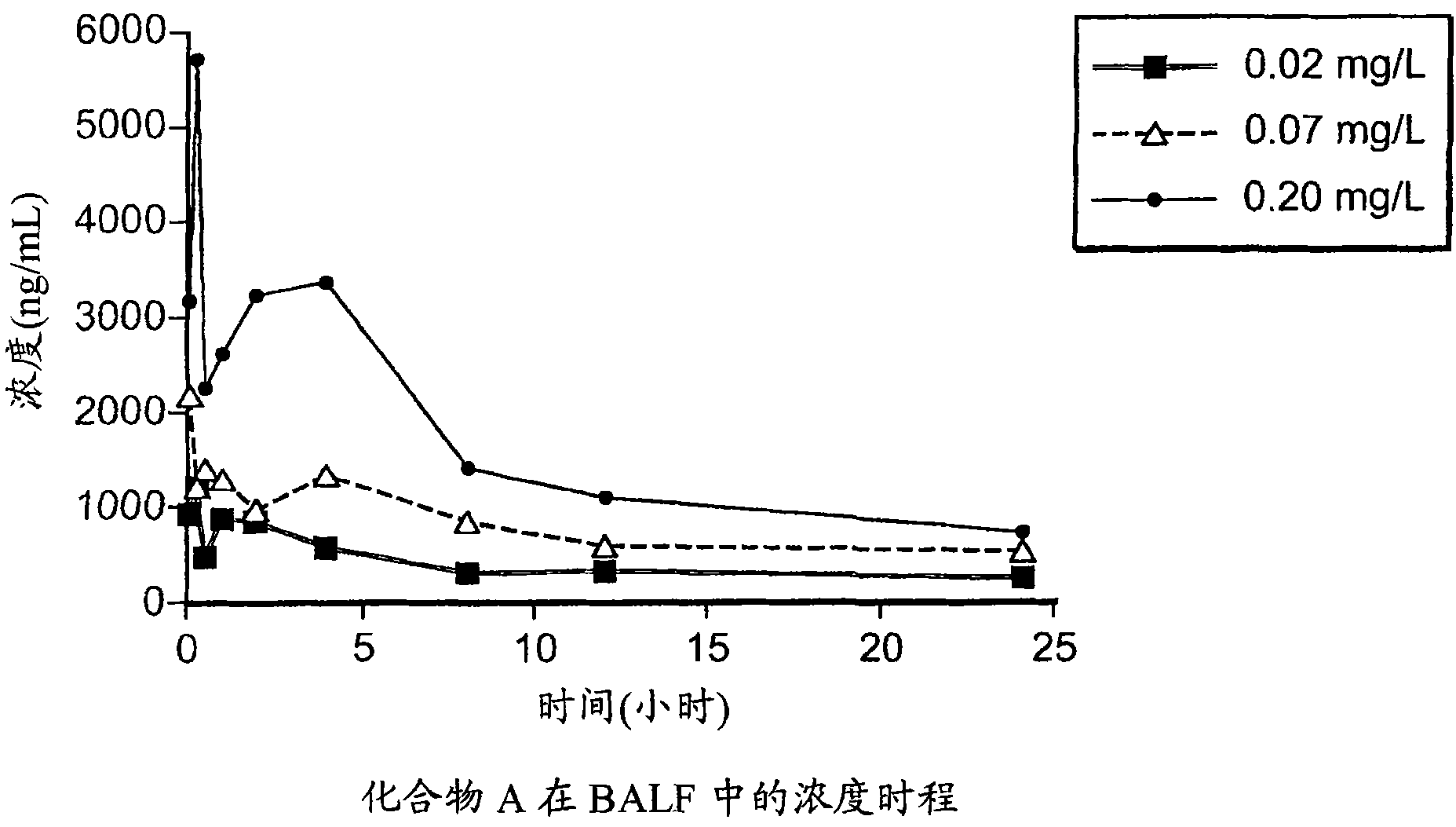
[0065] Use low concentration (0.02mg / L), middle concentration (0.07mg / L) and high concentration (0.20mg / L) target aerosol: 0.3% compound A / 0.075% tyloxapol, 0.6% compound A / 0.15% four Suspension of tyloxapol and 1.5% compound A / 0.375% tyloxapol. These low, medium and high target formulations were prepared as follows:
[0066] Remove 4% Compound A and 1% tyxapol from the freezer, transfer approximately 2.25, 4.5, and 12 mL into clean 50 mL graduated plastic tubes, labeled 0.3% A / 0.075% tyxapol, 0.6% A / 0.15% tyloxapol and 1.5% A / 0.375% tyloxapol suspension, allowe...
Embodiment 3
[0088] The lung exposure of embodiment 3 mice
[0089] A series of compositions for pulmonary delivery were prepared by preparing a 20% suspension of Compound A in 5% tyloxapol in water. use a colloid mill MK module, at 12,000 rpm for 6 hours while keeping the temperature below 50°C), resulting in particle size reduction. A 4% sample of Compound A was prepared by dilution with deionized water. A 0.4% suspension of Compound A in 0.1% tyloxapol solution was obtained by dilution with sterile water for injection (SWFI) (6.02 g of a 20% suspension was topped up to 30.23 g with sterile water for injection).
[0090] Prepare each of the following solutions as described below:
[0091] 0.4% compound A suspension in 0.1% tyloxapol solution: 2.07 g of a 4% compound A suspension was diluted to 20 g with deionized water.
[0092] 0.04% compound A suspension in 0.01% tyloxapol solution: 2.03 g of a 0.4% compound A suspension was diluted to 20 g with deionized water.
[0093] 0.004% c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com