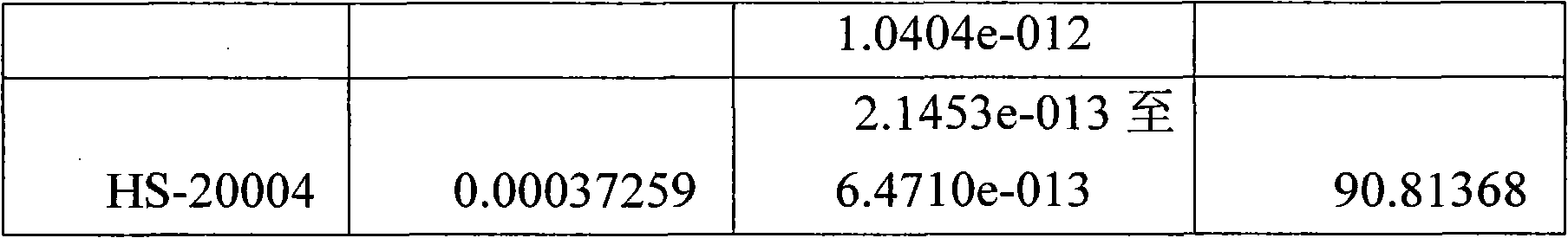Derivative or pharmaceutically acceptable salt of GLP-1 analogue and application of derivative or pharmaceutically-acceptable salt of a GLP-1 analogue
A technology of GLP-1 and analogues, applied in the field of derivatives of GLP-1 analogues or their pharmaceutically acceptable salts and applications, which can solve the problem of reduced activity of GLP-1 derivatives, suspension of clinical research, and unsuitability for chronic dosage strategies And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
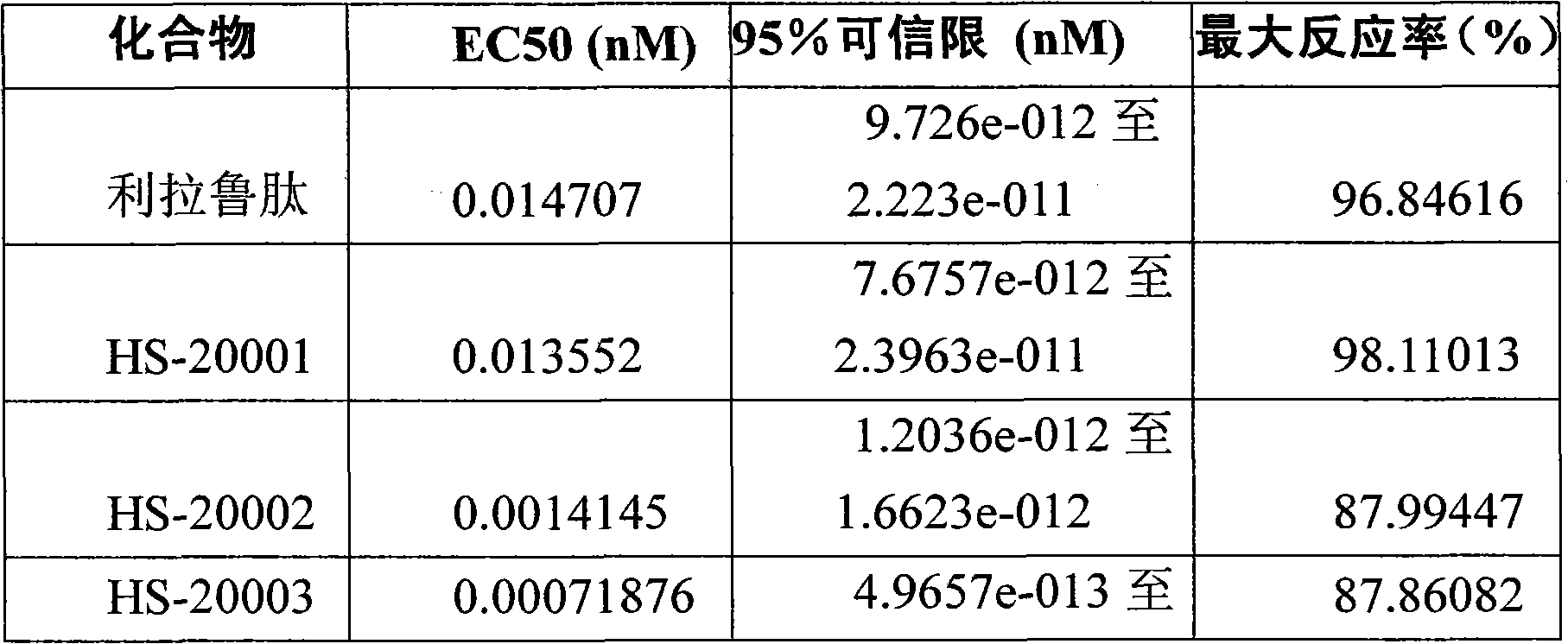
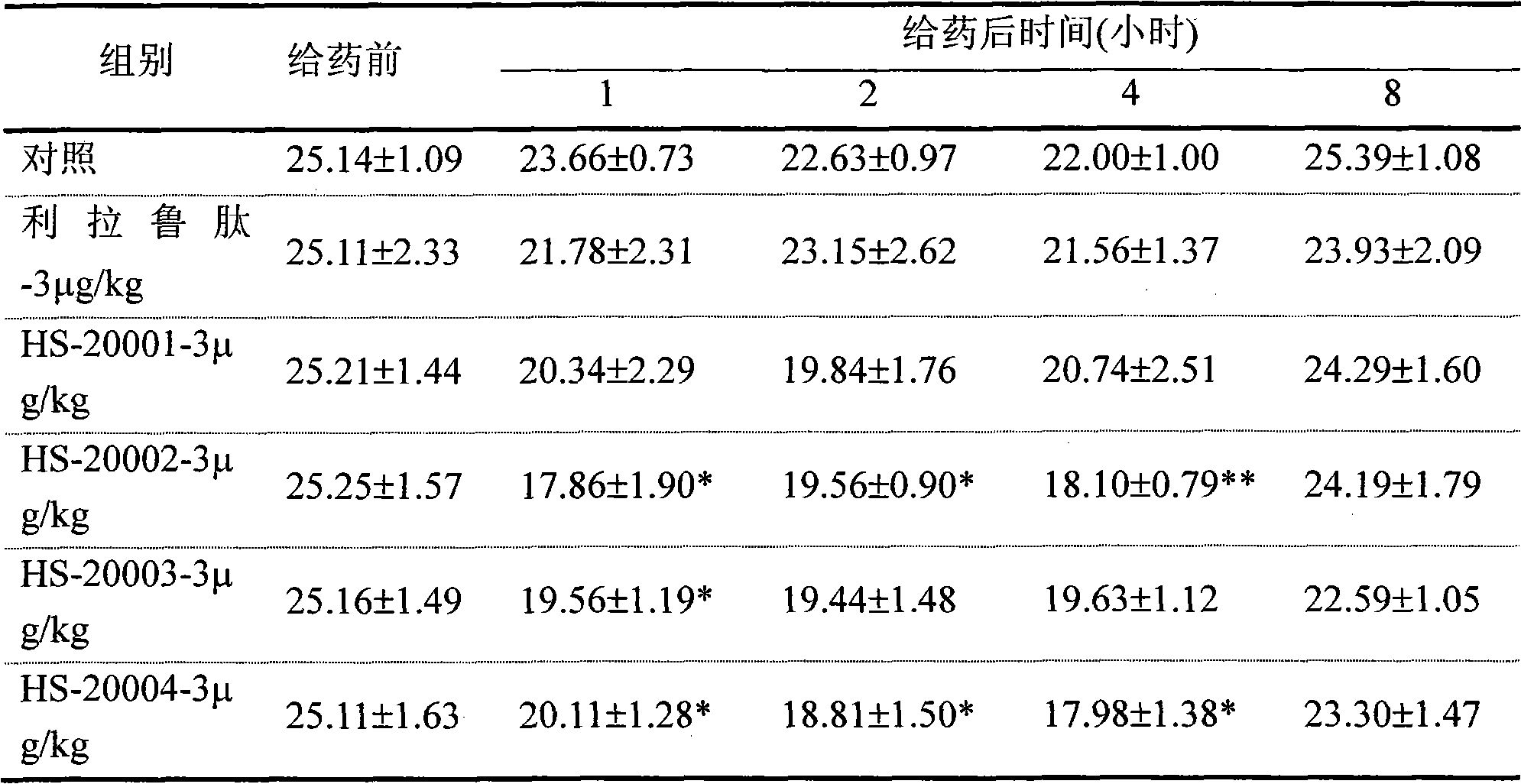
Embodiment 1
[0049] The solid phase synthesis method of embodiment 1 HS-20001
[0050] (1) Drying and swelling of solid phase synthetic resin
[0051] Weigh 50g (20mmol) of Fmoc-Lys(Mtt)-HMPA-AM resin (0.4mmol / g) which was vacuum-dried for 24 hours and place it in a 2L bubbler bottle, add 500mL N,N-dimethylformamide (DMF) Swell the resin for 30 minutes, then remove the DMF solution;
[0052] (2) Fmoc-Lys(Mtt)-HMPA-AM resin removes 4-methyltrityl (Mtt) protecting group
[0053] Wash the resin with 200mL DCM, repeat once, add 1200mL 1% TFA / DCM (TFA about 8 times excess) to remove the Mtt protecting group, reaction time 30 minutes, use 200mL 5% N, N-diisopropylethylamine (DIEA) / DMF and DMF were washed 3 times, and DMF was washed three times.
[0054] (3) Condensation of palmitic acid
[0055] Weigh 50mmol each of palmitic acid and 3-(diethoxyphosphoryloxy)-1,2,3-benzotriazin-4-one (DEPBT), add 400mL DMF to dissolve, then add 100mmol DIEA and stir the reaction at room temperature After 3...
Embodiment 2
[0070] The solid phase synthesis method of embodiment 2 HS-20002
[0071] (1) Drying and swelling of solid phase synthetic resin
[0072] Weigh 50g (20mmol) of Fmoc-Lys(Mtt)-HMPA-AM resin (0.4mmol / g) dried under vacuum for 24 hours and place it in a 2L bubbler bottle, add 500mL DMF to swell the resin for 30 minutes, and drain the DMF solution;
[0073] (2) Fmoc-Lys(Mtt)-HMPA-AM resin removes Mtt protecting group
[0074] Wash the resin with 200mL DCM, repeat once, add 1200mL 1% TFA / DCM (TFA about 8 times excess) to remove the Mtt protecting group, reaction time 30 minutes, wash 3 times with 200mL 5% DIEA / DMF and DMF, and wash 3 times with DMF .
[0075] (3) Condensation of palmitic acid
[0076] Weigh 50mmol each of palmitic acid and DEPBT, add 400mL DMF to dissolve, then add 100mmol DIEA, stir and react at room temperature for 3 minutes, add the above solution to the resin, and pass N 2 React for 2 hours. After the reaction was finished, the reaction solution was sucked ...
Embodiment 3
[0090] The solid-phase synthesis method of embodiment 3 HS-20003
[0091] (1) Drying and swelling of solid phase synthetic resin
[0092] Weigh 50g (20mmol) of Fmoc-Lys(Mtt)-HMPA-AM resin (0.4mmol / g) that was vacuum-dried for 24 hours and place it in a 2L bubbler bottle, add 500mL of DMF to swell the resin for 30 minutes, and remove the DMF solution;
[0093] (2) Fmoc-Lys(Mtt)-HMPA-AM resin removes Fmoc protecting group
[0094] Add 200mL of 20% piperidine / DMF solution to the bubble bottle filled with Fmoc-Lys(Mtt)-HMPA-AM resin, take it out after reacting for 5 minutes, then add 200mL of 20% piperidine / DMF solution and react at room temperature for 20 minutes. After the reaction, the resin was washed four times with 200 mL of DMF.
[0095] (3) Condensation of palmitic acid
[0096]Weigh 50mmol each of palmitic acid and DEPBT, add 400mL DMF to dissolve, then add 100mmol DIEA, stir and react at room temperature for 3 minutes, add the above solution to the resin, and pass N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com