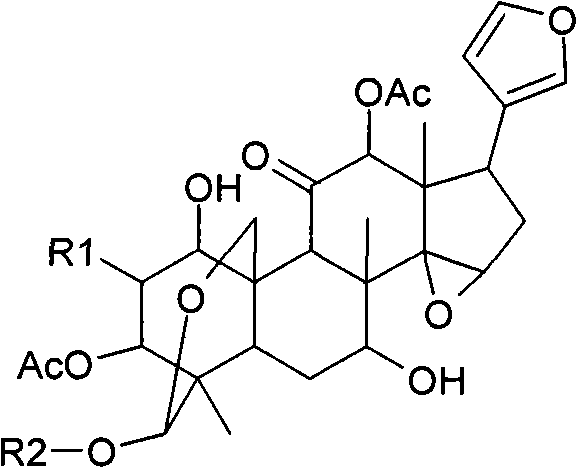
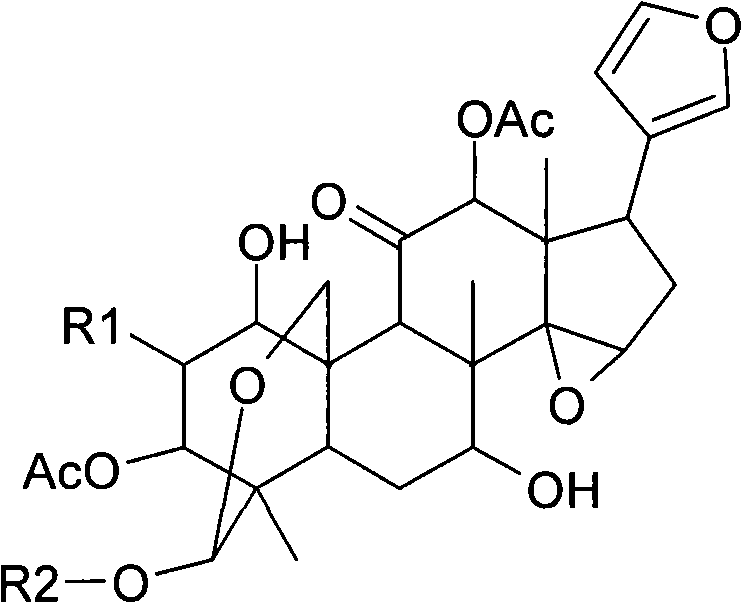
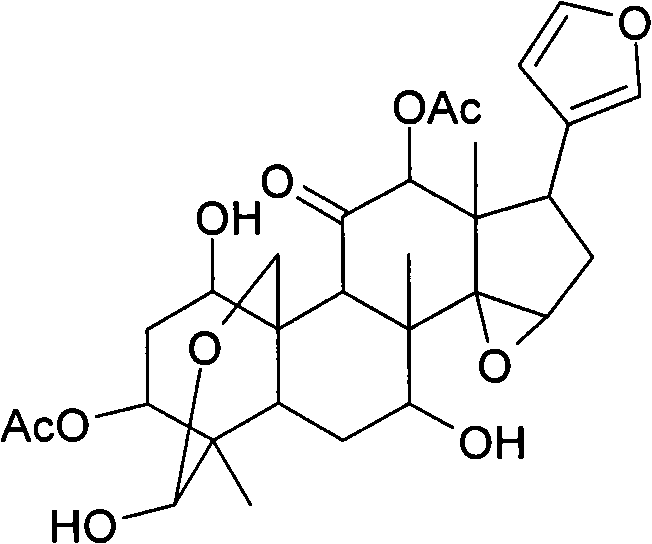
Anti-hepatitis C composition and method for preparing drugs for inhibiting hepatitis C virus or treating hepatitis C
A technology for hepatitis C virus and hepatitis C, which is applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as hepatitis C of unknown limonoid compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Extraction of effective components for inhibiting hepatitis C virus from plant materials
[0066] Dissolve 5.96g of toosendan alcohol extract in 20ml of mixed solvent of methanol and water, and extract 2-3 times with 15ml each of n-hexane, diethyl ether, dichloromethane and ethyl acetate respectively to obtain n-hexane layers extract, diethyl ether layer extract, dichloromethane layer extract and ethyl acetate layer extract. The extracts of each layer were concentrated and dried to obtain the n-hexane layer extract, the ether layer extract, the dichloromethane layer extract and the ethyl acetate layer extract respectively, and these extracts were respectively treated with Huh-luc / neo-ET cells ( HCV Cell Replication System) to test the activity of these extracts against HCV.
[0067] The results show that the gained ether layer extract (1.113g) has the activity of inhibiting the replication of hepatitis C virus, and its inhibitory concentration (IC) to 50% hepatitis C v...
Embodiment 2
[0080] 1. Toxicity test of toosendanin on Huh-luc / neo-ET cells
[0081] Huh-luc / neo-ET cells in 2.5×10 4 Cells / 100 μl / well were seeded in 96-well cell culture plates (Corning Incorporation COSTAR, 3599) and cultured in a cell culture incubator (NUAIR nu-5510).
[0082] The next day, dilute the toosendanin samples with DMEM culture solution to concentrations of 28.73μg / ml, 9.57μg / ml, 3.19μg / ml, 1.06μg / ml, 0.35μg / ml, 0.11μg / ml, 0.039μg / ml Different from 0.013μg / ml, or diluted to 114.92μg / ml, 38.33μg / ml, 12.77μg / ml, 4.25μg / ml, 1.42μg / ml, 0.46μg / ml, 0.16μg / ml and 0.057μg / ml Toosendanin culture solution with diluted concentration, and the original culture solution in the 96-well plate was sucked off by a vacuum pump (DOAT-704AA). During this process, care must be taken not to suck the cells. Then add toosendanin culture solution of the above concentration in the amount of 100 μl / well to the above-mentioned 96-well cell culture plate containing cells as the experimental group, and...
Embodiment 3
[0094] 1. Toxicity test of Trinortriterpene H (Trichilin H) on Huh-luc / neo-ET cells
[0095] The experimental procedure is the same as in Example 2, and the test sample is replaced by tetranortriterpene H. Among them, tetranortriterpene H samples were diluted with DMEM culture medium to concentrations of 50 μg / ml, 25 μg / ml, 8.33 μg / ml, 2.78 μg / ml, 0.93 μg / ml, 0.31 μg / ml, 0.1 μg / ml and 0.03 μg / ml tetranortriterpene H culture solution.
[0096] The results are shown in Table 1. The 50% lethal concentration of tetranortriterpene H to cells (CC 50 ) greater than 50μg / ml, 15% lethal concentration (CC 15 ) was 0.7 μg / ml.
[0097] 2. Test the fluorescence activity of fireflies to evaluate the effect of trinortriterpene H (Trichilin H) on inhibiting the replication of hepatitis C virus
[0098] The experimental procedure is the same as in Example 2, and the test sample is replaced by tetranortriterpene H. Huh-luc / neo-ET cells were co-cultured with tetranortriterpene H at concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com