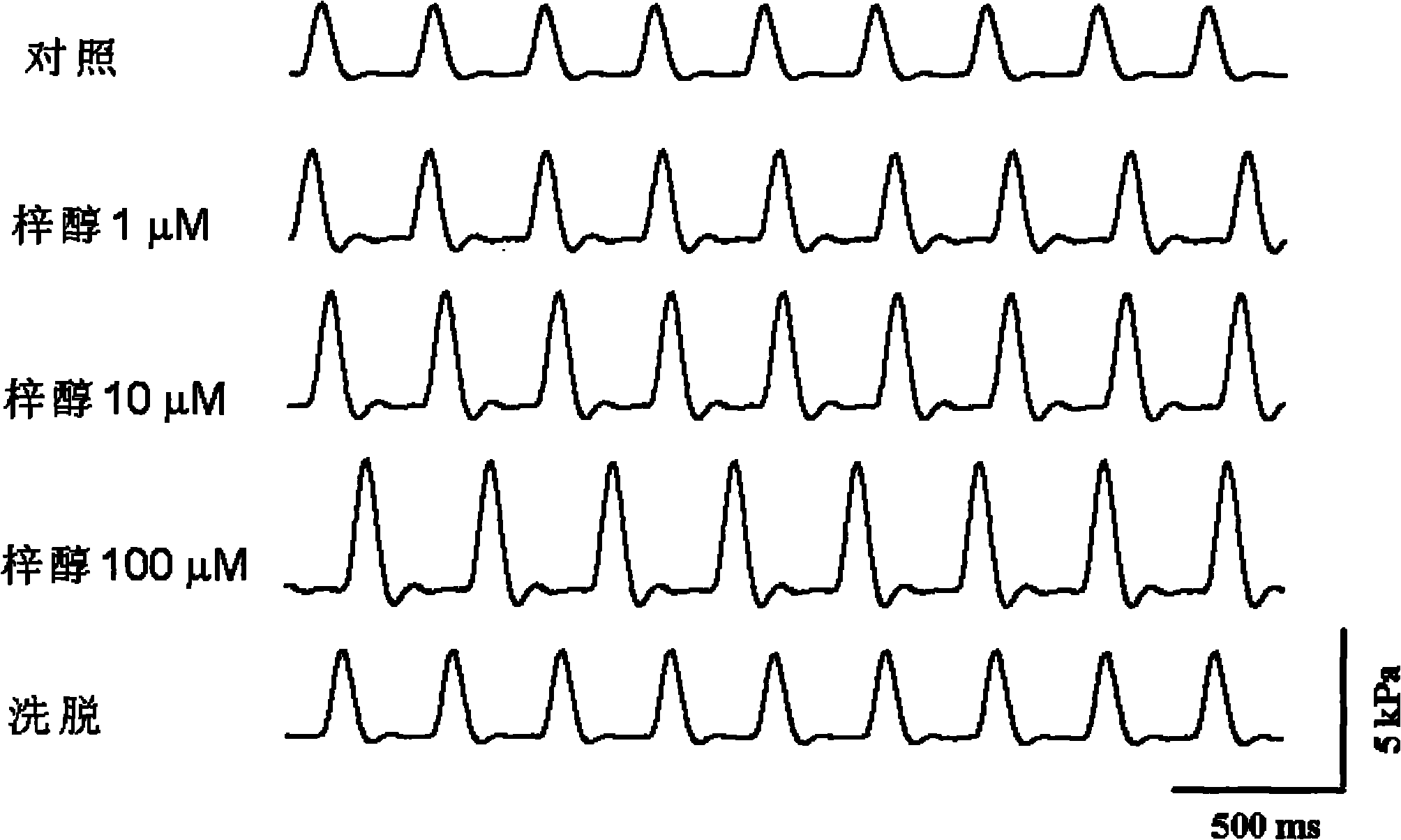
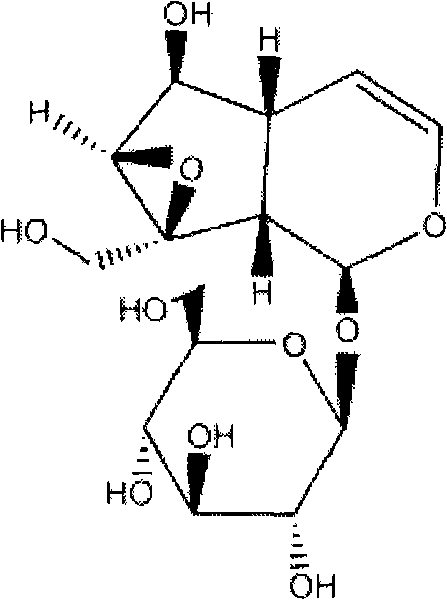
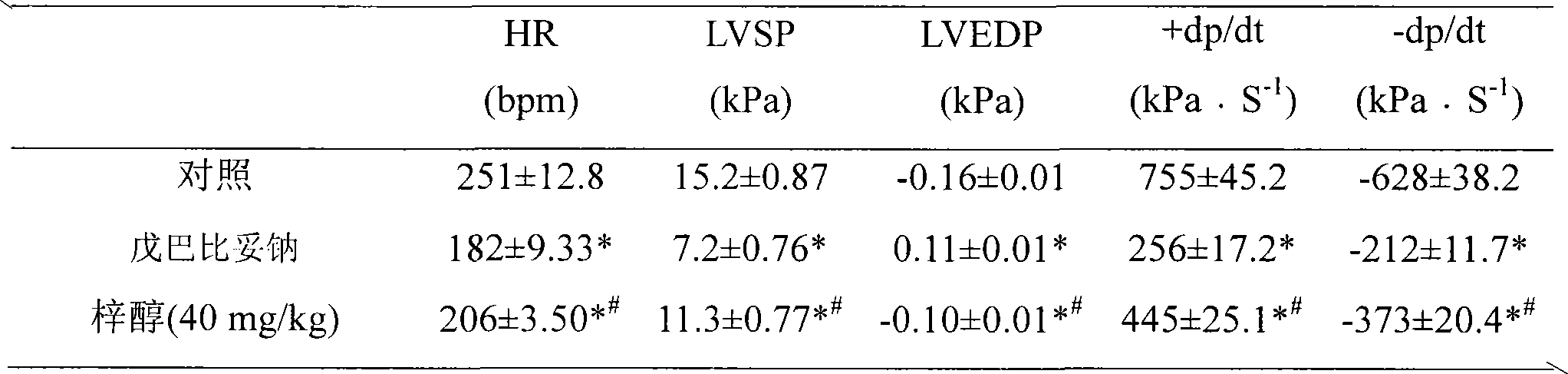
Application of catalpol in preparing drug for treating cardiac failure disease
A heart failure, drug technology, applied in cardiovascular system diseases, drug combinations, pharmaceutical formulations, etc., can solve problems such as damage to organs, toxic side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0015] Preparation Example 1: Preparation of Catalpol Injection
[0016] Weigh 25.0g of catalpol raw material, dissolve in 1000ml of water for injection, fully stir to dissolve, add water for injection to 2000ml, add 2.5g of activated carbon for needles, heat to 60°C and stir for 30 minutes, filter with carbon rod, and filter the filtrate through 0.25μm Sterilize by filtration with a microporous membrane, pack in 1000 vials, with a filling volume of 2.0ml / bottle, seal, and sterilize. Each bottle contains catalpol 20mg. The adult body weight is based on 60kg, and the clinical dosage of catalpol injection for adults is 0.8mg / kg / d of catalpol.
preparation example 2
[0017] Preparation example 2: Preparation of catalpol freeze-dried powder injection
[0018] Weigh 25.0g of catalpol raw material and 50.0g of medicinal mannitol, dissolve in 1000ml of water for injection, stir well to dissolve, add water for injection to 2000ml, add 2.5g of activated carbon for needles, heat to 60°C and stir for 30 minutes, carbon Rod filtration, the filtrate is sterilized by filtration through a 0.25 μm microporous membrane, divided into 1000 vials, the filling volume is 2.0ml / vial, freeze-dried, and obtained. Each bottle contains catalpol 20mg.
experiment example 4
[0034] Experimental Example 4 Catalpol Acute Toxicity Test
[0035] Get 20 healthy KM mice, 18-22g, half male and half male, inject catalpol maximum amount 8mg / 20g (mouse body weight) into the tail vein, after a single administration, observe the abnormal reaction that occurs in the animal within 7 days, the death situation and cause of death. Results: After the catalpol injection was injected into the tail vein of the mice, the activity of the tested animals decreased significantly within 2 hours after the administration, and the general condition was good during the observation period thereafter, with no decrease in activity, no decrease in food intake, and no loose stools . The animals were sacrificed 7 days later, and no pathological changes visible to the naked eye were found on dissection. The test results show that the maximum administration dose of catalpol for mouse tail vein injection is 400mg / kg, and the catalpol is made into traditional Chinese medicine injection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com