Eye-drop preparation and use thereof
一种滴眼剂、制剂的技术,应用在含有效成分的医用配制品、药物输送、有机活性成分等方向,能够解决分解等问题,达到提高稳定性、降低药物反应、含量降低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
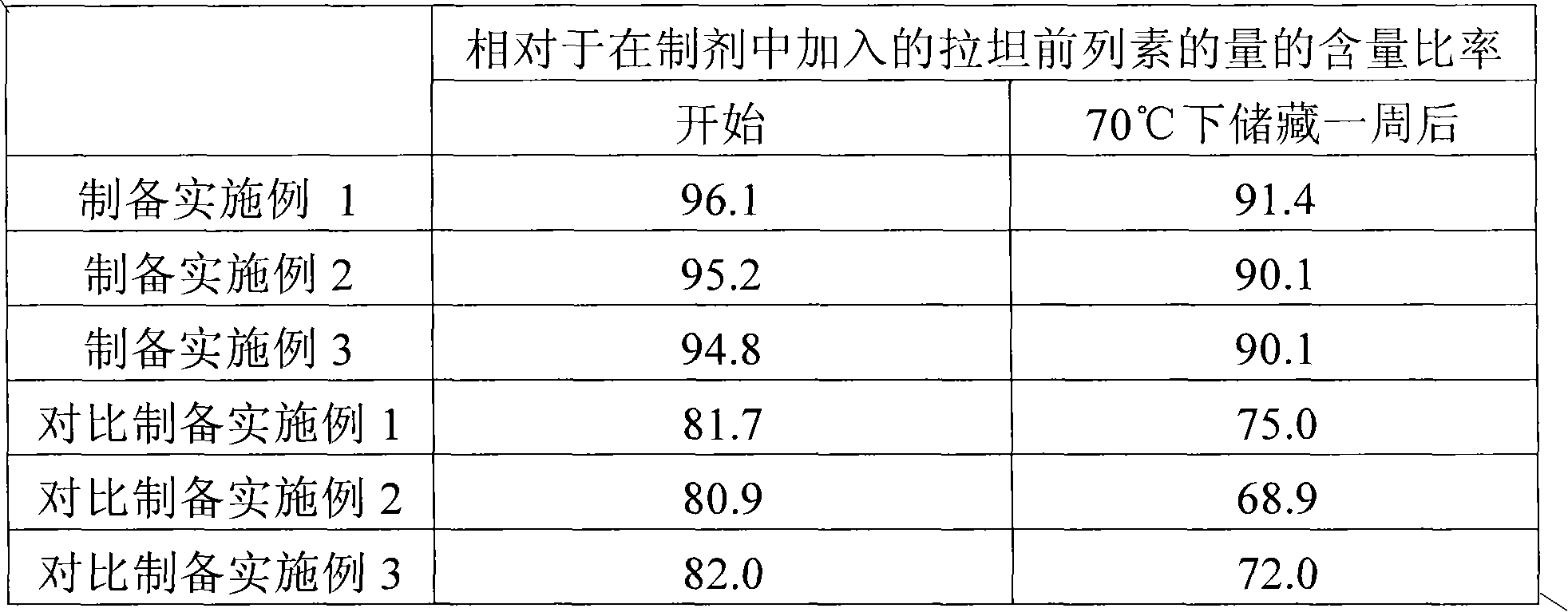
preparation Embodiment 1
[0041] Take by weighing 2g carteolol hydrochloride, 5.2mg latanoprost, 0.04g sodium dihydrogen phosphate dihydrate, 0.1g disodium hydrogen phosphate dodecahydrate and 0.54g sodium chloride, and in it Add about 80ml of purified water. The mixture was stirred while heating to about 60°C to dissolve. After returning it to room temperature, the resulting solution was made up to 100 ml by adding purified water thereto, thereby preparing an eye drop formulation with a pH of 6.8.
preparation Embodiment 2
[0043] An eye drop formulation having a pH value of 6.8 was prepared in the same manner as in Preparation Example 1 except that 5 mg of germine was added to the composition of Preparation Example 1.
preparation Embodiment 3
[0045] An eye drop preparation having a pH of 6.8 was prepared in the same manner as in Preparation Example 1 except that 20 mg of germilamine was added to the composition of Preparation Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com