Serum/plasma miRNA composition and use thereof
A technology of composition and primer composition, applied in the field of molecular biology, can solve problems such as incomplete clarification of the relationship, and achieve the effects of quickly and accurately grasping the patient's condition, being easy to obtain, and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preliminary screening of specific miRNA expression profile in patients with chronic hepatitis B (Solexa sequencing entrusted Shenzhen Huada Gene Research Institute to complete)
[0067] Using Solexa sequencing technology to discover and prove that there are 88 differentially expressed microRNAs in the serum / plasma of 30 healthy controls and 30 chronic hepatitis B patients. The specific steps are:
[0068] (1) Collect serum / plasma from healthy controls and chronic hepatitis B patients;
[0069] (2) Take 80-100ml of serum respectively and add an equal volume of Trizol Reagent;
[0070] (3) Phase separation: place at room temperature for 15 minutes, then add chloroform according to the volume ratio of 0.2ml chloroform / 1ml Trizol Reagent, shake for 15s in the education column, 15 minutes at room temperature, 12,000g, 4°C, centrifuge for 15 minutes;
[0071] (4) Transfer the aqueous phase to a new 50ml centrifuge tube, and remove the protein phase in 3 steps of p...
Embodiment 2
[0085] Example 2 Real-time PCR verification was performed on the miRNAs initially screened by Solexa technology, and a group of miRNAs with stable and significant differences in expression were screened out
[0086] qRT of microRNAs was performed on sera from 30 healthy controls (Solexa sequencing samples), 30 virus-cleared individuals, 30 asymptomatic HBV-infected patients, 30 chronic hepatitis B patients (Solexa sequencing samples), and 30 chronic hepatitis C patients - PCR test.
[0087] (1) Preparation of cDNA samples: a) Take 500 μL of serum; b) Add an equal volume of water-saturated phenol, shake and mix, centrifuge at 13200 rpm for 3 minutes at 4°C, and take the supernatant; c) Supernatant + 1 / 2 volume (250 μL ) Phenol + 1 / 2 volume (250μL) chloroform, shake and mix, 4°C, centrifuge at 13200rpm for 3 minutes, take the supernatant; Take the supernatant as an RNA sample; e) Then obtain cDNA through RNA reverse transcription reaction. The reverse transcription reaction sy...
Embodiment 3
[0089] After the samples in Example 3 were reintegrated and the sample size was enlarged, the group of miRNAs screened in Example 2 was verified by quantitative PCR method, and the application and clinical value of this group of miRNAs in the process of HBV positive diagnosis were evaluated.
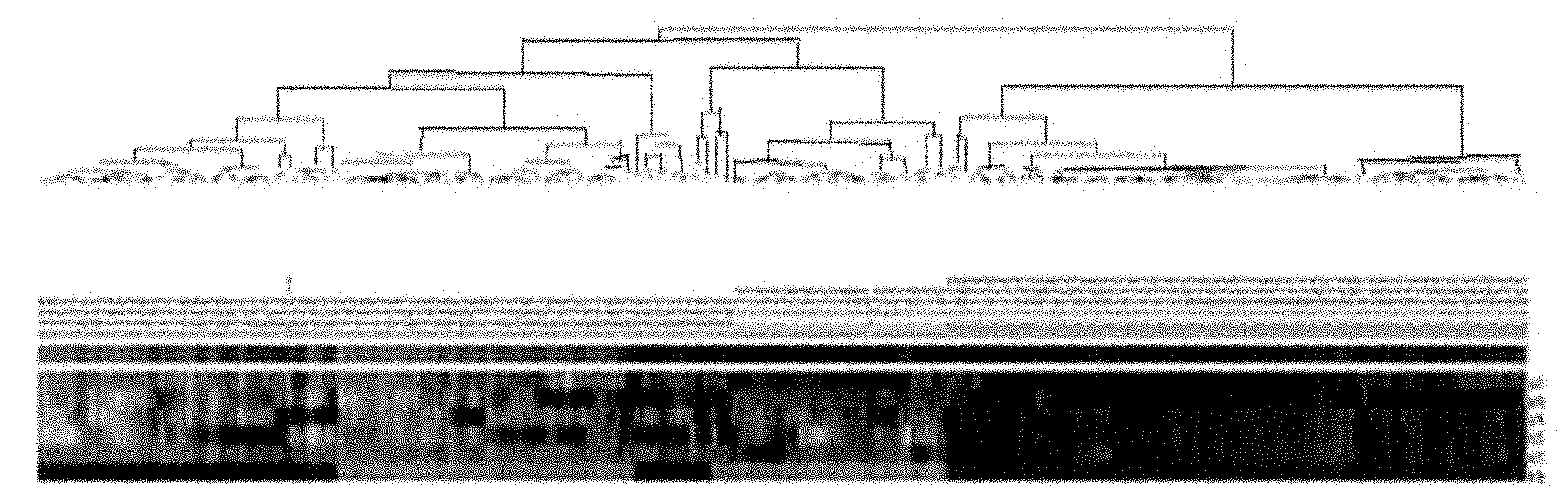
[0090] Serum of 100 cases of control group (50 cases of healthy controls and 50 cases of virus clearance), 75 cases of HBV group (25 cases of HBV carriers and 50 cases of chronic hepatitis B patients) and 18 cases of patients with chronic hepatitis C group were tested for microRNA. qRT-PCR test. The experimental method, qRT-PCR result processing method and clustering method are the same as in Example 2.
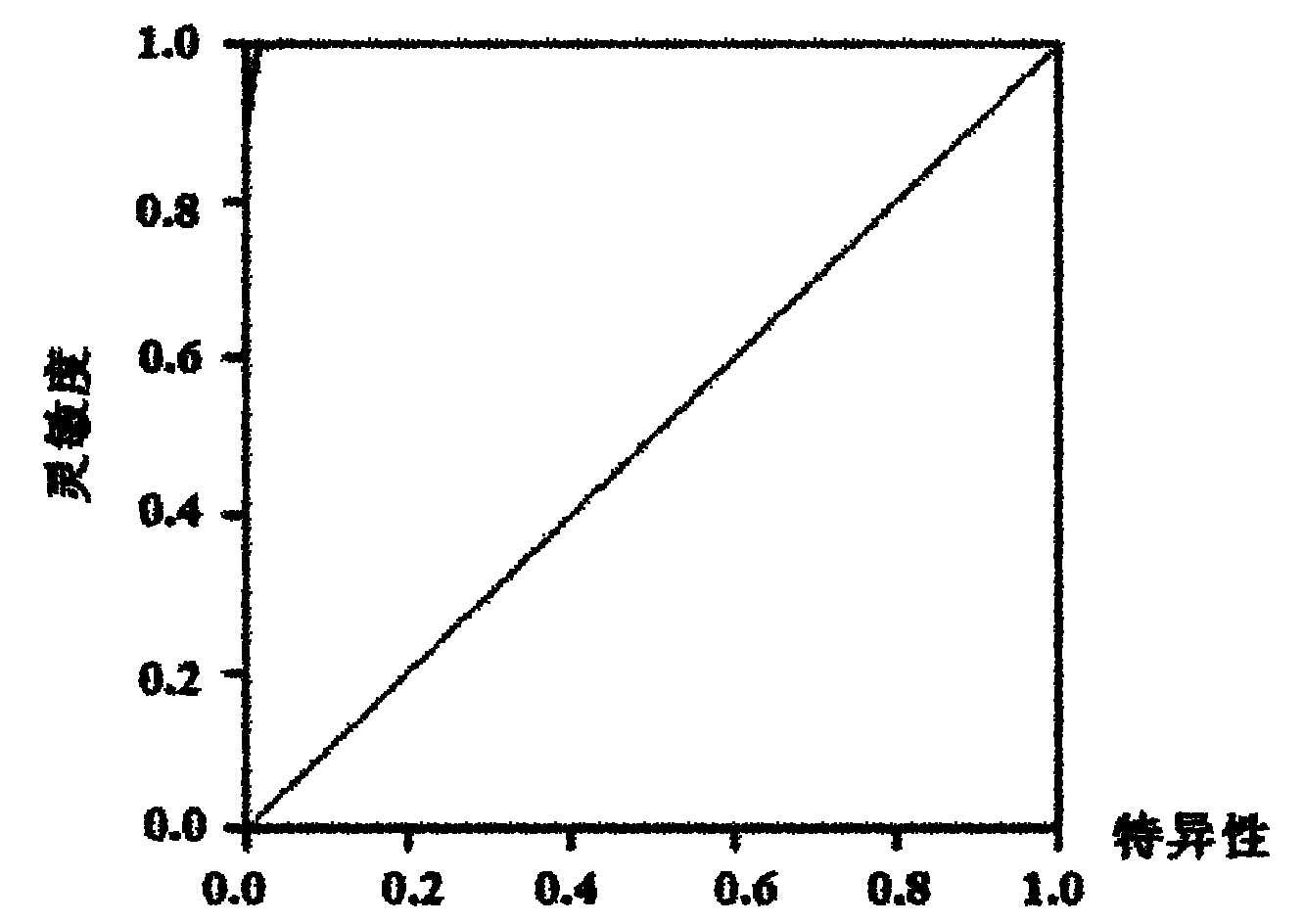
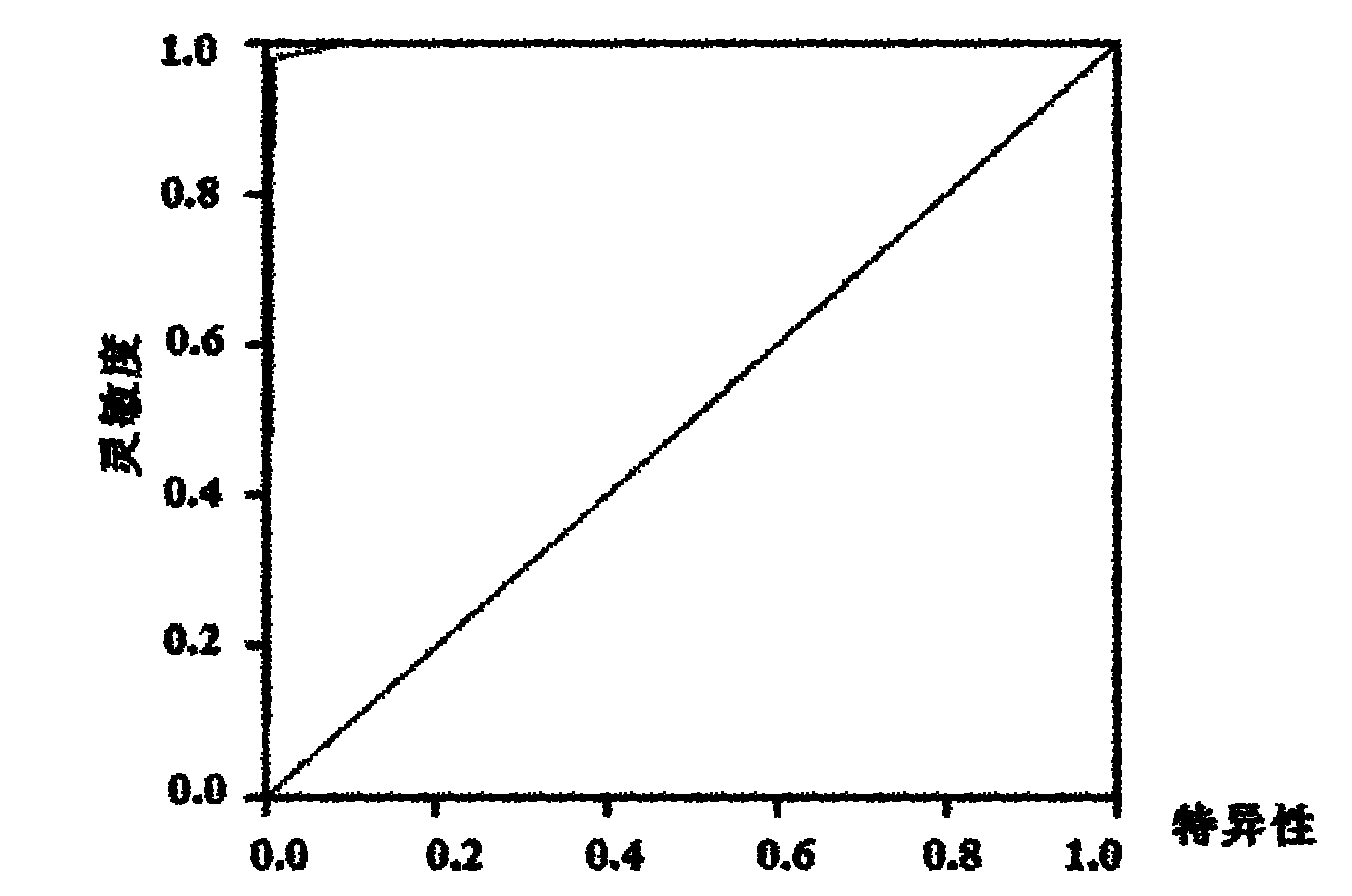
[0091]The results of qRT-PCR further proved that there is no significant difference between healthy controls and virus cleared persons, between HBV carriers and chronic hepatitis B patients. hsa-miR-122, hsa-miR-423-5p, hsa-miR-92a, hsa-let-7c, hsa-miR-23a, hsa-miR-23b, hsa-miR-223, hs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com