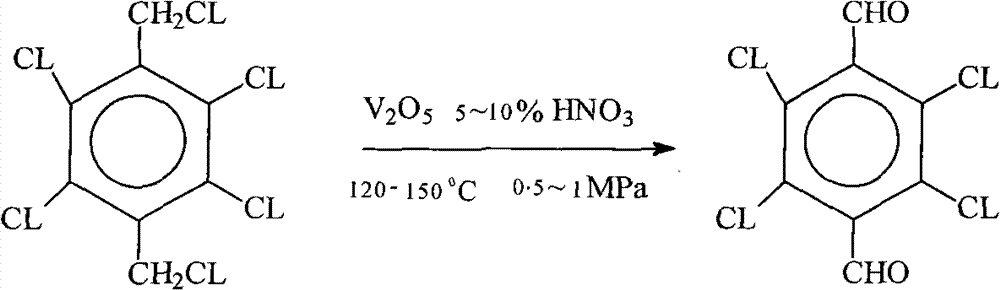Preparation method of 2,3,5,6-tetrachloro-terephthalaldehyde
A technology of tetrachloroterephthalaldehyde and tetrachloro-p-phenylene dichlorobenzyl, which is applied in two fields, can solve the problems of low yield and expensive raw materials, and achieve the effects of low production cost, easy operation, and no waste acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 31.3 grams of 2,3,5,6-tetrachloro-p-benzenedichlorobenzyl, 600 grams of dilute nitric acid with a weight concentration of 5% and 1.5 grams of vanadium pentoxide into the autoclave, and then put it at 150°C and 0.8MPa pressure The oxidation reaction is 20 hours. Cool, filter, then reconsolidate with acetone and water. After drying, 23 g of 2,3,5,6-tetrachloroterephthalaldehyde was obtained, and the yield was about 85%. The mother liquor can be recycled after removing HCL by adding nitric acid.
Embodiment 2
[0021] Add 31.3 grams of 2,3,5,6-tetrachloro-p-phenylenedichlorobenzyl, 400 grams of dilute nitric acid with a weight concentration of 10% and 1 gram of vanadium pentoxide into the autoclave, and then at 120°C and 0.25MPa pressure Oxidize for 20 hours. Cool, filter, then reconsolidate with acetone and water. After drying, 20.5 g of 2,3,5,6-tetrachloroterephthalaldehyde was obtained, and the yield was about 75%. The mother liquor can be recycled after removing HCL by adding nitric acid.
Embodiment 3
[0023] Add 31.3 grams of 2,3,5,6-tetrachloro-p-phenylene dichlorobenzyl, 600 grams of 8% dilute nitric acid by weight and 1.5 grams of vanadium pentoxide into the autoclave, and then oxidize it at 150°C and 0.5MPa pressure. hour. Cool, filter, then reconsolidate with acetone and water. After drying, 24 g of 2,3,5,6-tetrachloroterephthalaldehyde was obtained, and the yield was about 88%. The mother liquor can be recycled after removing HCL by adding nitric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com