Method for detecting dissolution rates of acetylkitasamycin capsules
A technology for the detection of acetylkitamycin and its detection method, which is applied in the field of detection of the dissolution rate of acetylkitamycin capsules. It can solve the problems of unbreakable swelling of the preparation, affecting the measurement, and the dissolution measurement method needs to be optimized, so as to overcome the problem of reduced release in vitro. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 Acetylkitamycin Capsules Dissolution Determination
[0020] Preparation of pepsin solution: Take 16.4mL of dilute hydrochloric acid with a mass percentage concentration of 9.5-10.5%, add about 800mL of water and 1:15,000 units of pepsin (15,000 units of pepsin activity per 1g) 6-12g or 1:3000 units of pepsin (Each 1g of pepsin activity is 3000 units) 10g, dissolve and shake well, add water to dilute to 1000mL, that is.
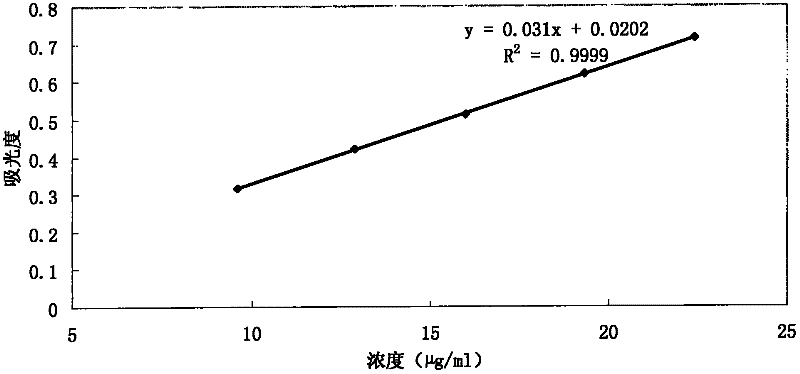
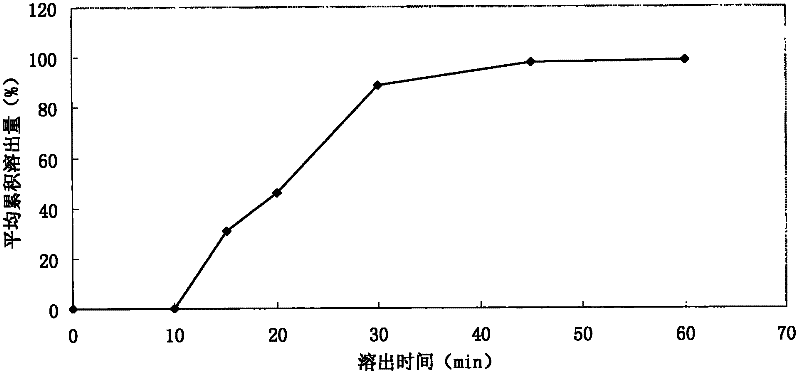
[0021] Preparation of the test solution: get 6 capsules of acetylkitasamycin (about 200,000 units / grain of acetylkitasamycin), take 900mL of pepsin solution as the dissolution medium, adopt the second method of Appendix XC of the Chinese Pharmacopoeia in 2010, The rotating speed is 100 revolutions per minute. When operating according to the law for 60 minutes, take an appropriate amount of the solution, filter it, and accurately measure 3 mL of the subsequent filtrate and put it in a 50 mL measuring bottle. Contains 12 to 15 units of a...
Embodiment 2
[0035] Embodiment 2 is the dissolution rate measurement when taking hydrochloric acid solution as dissolution medium
[0036] Take 6 capsules of acetylkitasamycin capsules (200,000 units / capsule), and use the slurry method for determination, use 900mL hydrochloric acid solution as the dissolution medium, and the rotation speed is 100 revolutions per minute. When operating according to the law to 60 minutes, take an appropriate amount of the solution, filter , accurately measure 3mL of the continued filtrate and put it in a 50mL measuring bottle, use 1.56-1.72‰ hydrochloric acid solution to make up the volume, and dilute it into an acetylkitamycin solution containing about 13 units per 1mL; another amount equivalent to about 167000 One unit of acetylkitasamycin capsule content, dissolved with 1.56-1.72‰ hydrochloric acid solution and diluted to 250mL, took 2mL of the above dilution solution into a 100mL measuring bottle, added 6mL of filtered pepsin solution, diluted with 1.56-1...
Embodiment 3
[0039] The impact of embodiment 3 pepsin addition on dissolution rate measurement
[0040] Take 6g, 8g, 10g, and 12g of pepsin with an activity of 1:15000, dissolve them in hydrochloric acid solution with a mass percentage concentration of 1.56-1.72‰, and dilute to 1000mL to obtain dissolution media containing different pepsin solubility, which are respectively recorded as medium A , Medium B, Medium C, and Medium D, respectively use dissolution medium A, medium B, medium C, and medium D to measure the dissolution rate of acetylkitamycin capsules according to the method of Example 1. The measurement results are shown in Table 3
[0041] Table 3: The effect of the amount of pepsin on the dissolution test
[0042]
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com