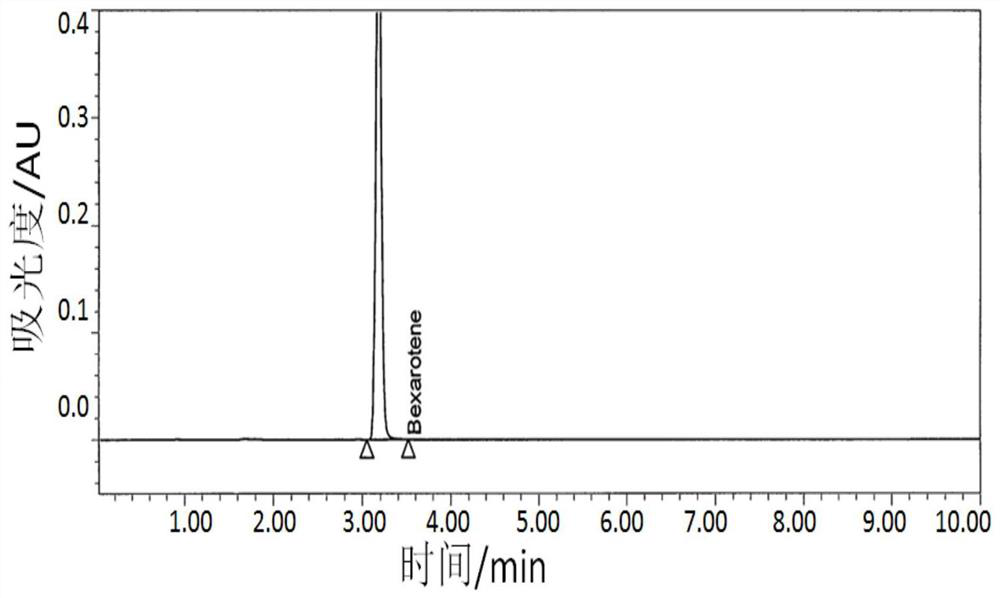
A kind of method for measuring the dissolution of besarotene soft capsules with enzyme
A technology of soft capsule and dissolution medium, applied in the field of medicine, can solve the problems of unbreakable swelling of preparations, difficult to release, cross-linking and combination of soft capsules, etc., and achieve the effect of overcoming the reduction of in vitro release degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: The method for measuring the dissolution of besarotene soft capsules with enzyme
[0032] (1) Preparation of phosphate buffer solution: prepare 2100ml of 0.05M potassium dihydrogen phosphate solution and 450ml of 0.2M sodium hydroxide solution, add water to 6 liters, and adjust the pH to 7.5±0.05 with phosphoric acid or sodium hydroxide;
[0033] Prepare dissolution medium A with the above-mentioned phosphate buffer: add 80.0 mg of pancreatin to 4 liters of phosphate buffer, stir evenly;
[0034] Prepare dissolution medium B with the above phosphate buffer: add 30g of HDTMA to 2 liters of phosphate buffer, and stir at 37°C for 1 hour;
[0035] (2) run the dissolution apparatus: the setting condition is the slurry method, the dissolution speed is 75rpm / min, the temperature is 37°C, and the dissolution time is 45 minutes;
[0036] (3) Preparation of the test solution: add 600ml of dissolution medium A into the dissolution vessel, run the instrument to 37°C, ad...
Embodiment 2
[0046] Example 2: Dissolution Methodology Study
[0047] 1. Step-by-step addition of dissolution speed and dissolution medium: The amount of pancreatin added in dissolution medium A was 20 mg / L, the dissolution time was 45 min, the dissolution volume was 900 ml, and the speed of dissolution was 50 rpm / min, 75 rpm / min, and 100 rpm / min, respectively. The dissolution rate of medium A and dissolution medium B mixed and added and added in steps, the results are shown in Table 1, where 900ml (A+B) refers to the one-step addition of the dissolution medium, and the dissolution medium includes the phosphate buffer solution of the present invention. , trypsin and HDTMA, the preparation method is as follows: add 80 mg of pancreatin and 30 g of HDTMA to 6 L of phosphate buffer, and stir at 37° C. for 1 h.
[0048] Table 1 The results of the step-by-step addition of the dissolution speed and the dissolution medium
[0049]
[0050] The results showed that the soft capsules did not diss...
Embodiment 3
[0059] Example 3: HPLC method validation
[0060] 1. Linear
[0061] 1.1 Preparation of reference stock solution
[0062] Weigh 80mg of besarotene compound standard into a 100ml volumetric flask (concentration is 800ug / mL), first dissolve with 30ml of methanol, and then use dissolution medium (the volume ratio of dissolution medium A to dissolution medium B is 2:1) Volume.
[0063] 1.2 Precisely pipet the reference stock solution according to Table 4
[0064] Table 4 Pipetting of reference substance stock solution
[0065] sample Volume of Control Stock Solution Constant volume Concentration (ug / mL) 1 2mL 100mL 16 2 4mL 100mL 32 3 4mL 50mL 64 4 5mL 50mL 80 5 15mL 100mL 120
[0066] 1.3 The linearity measured by HPLC is y=26835.929x+6749.964, R 2 =1.000.
[0067] 2. Precision
[0068] According to the dissolution method of the present invention, 6 besarotene soft capsules were randomly selected, and the dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com