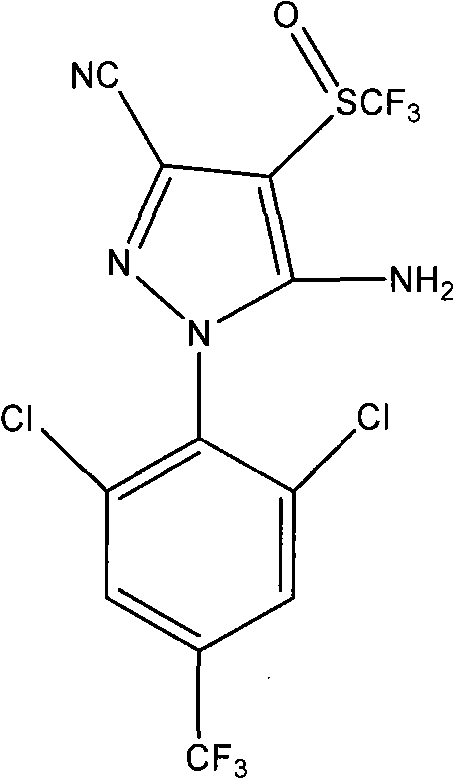
Method for synthesizing fipronil
A synthetic method, the technology of fipronil, which is applied in the field of synthesis of fipronil, can solve the problems of high toxicity of trifluoromethylsulfur chloride, low yield and high requirements for operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] Method steps of the present invention are:
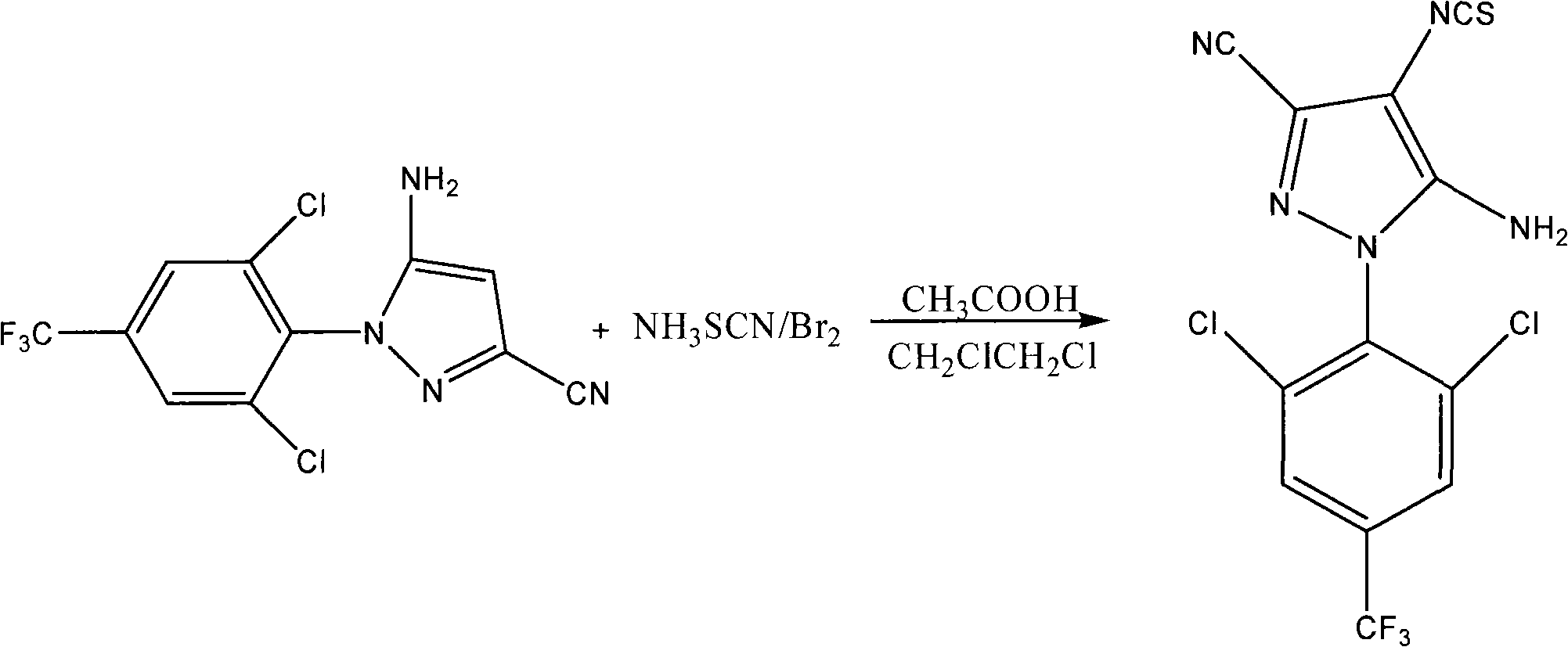
[0028] 1. Synthesis of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-4-thiocyano-pyrazole
[0029] The reaction equation is:
[0030]
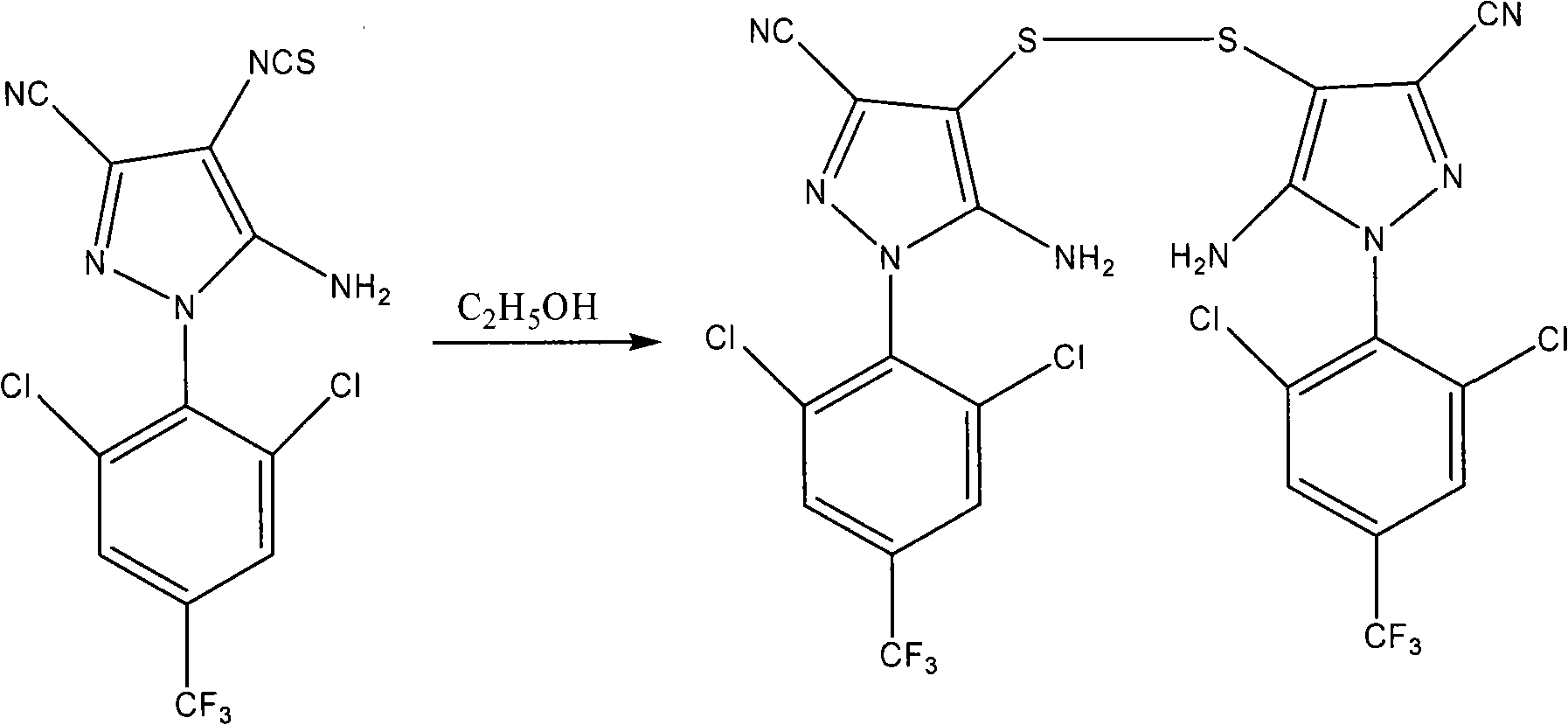
[0031] 2. Synthesis of Disulfides
[0032] The reaction equation is:
[0033]
[0034] 3. Synthesis of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-phenyl)-4-trifluoromethylthio-pyrazole
[0035] The reaction equation is:
[0036]
[0037] 4. Synthesis of Fipronil
[0038] The reaction equation is:
[0039]
[0040] The structure of the final product of the present invention has been confirmed by infrared spectrum and nuclear magnetic spectrum, and the spectral data are as follows: IR 1761 (C=O), 1697 (C=C), 3068 (C-H), 1637 (C-N).
[0041] HNMR (CDCl 3 , 400MHz) 1.11 (s, 6H, CH 3 ), 3.57 (s, 4H, CH 2 ), 8.53 (s, 1H, pY-H).
[0042] 13 CNMR (CDCl 3 , 100MHz) 16.2, 48.1, 120.6, 126.1, 139.4, 140.8, 151.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com