Fusion protein immunosuppressive agent and preparation method and application thereof
A fusion protein and fusion gene technology, applied in the fields of biotechnology and medicine, can solve the problems of low efficiency of immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The construction of embodiment 1 fusion protein sHLA-G / IgG eukaryotic expression vector
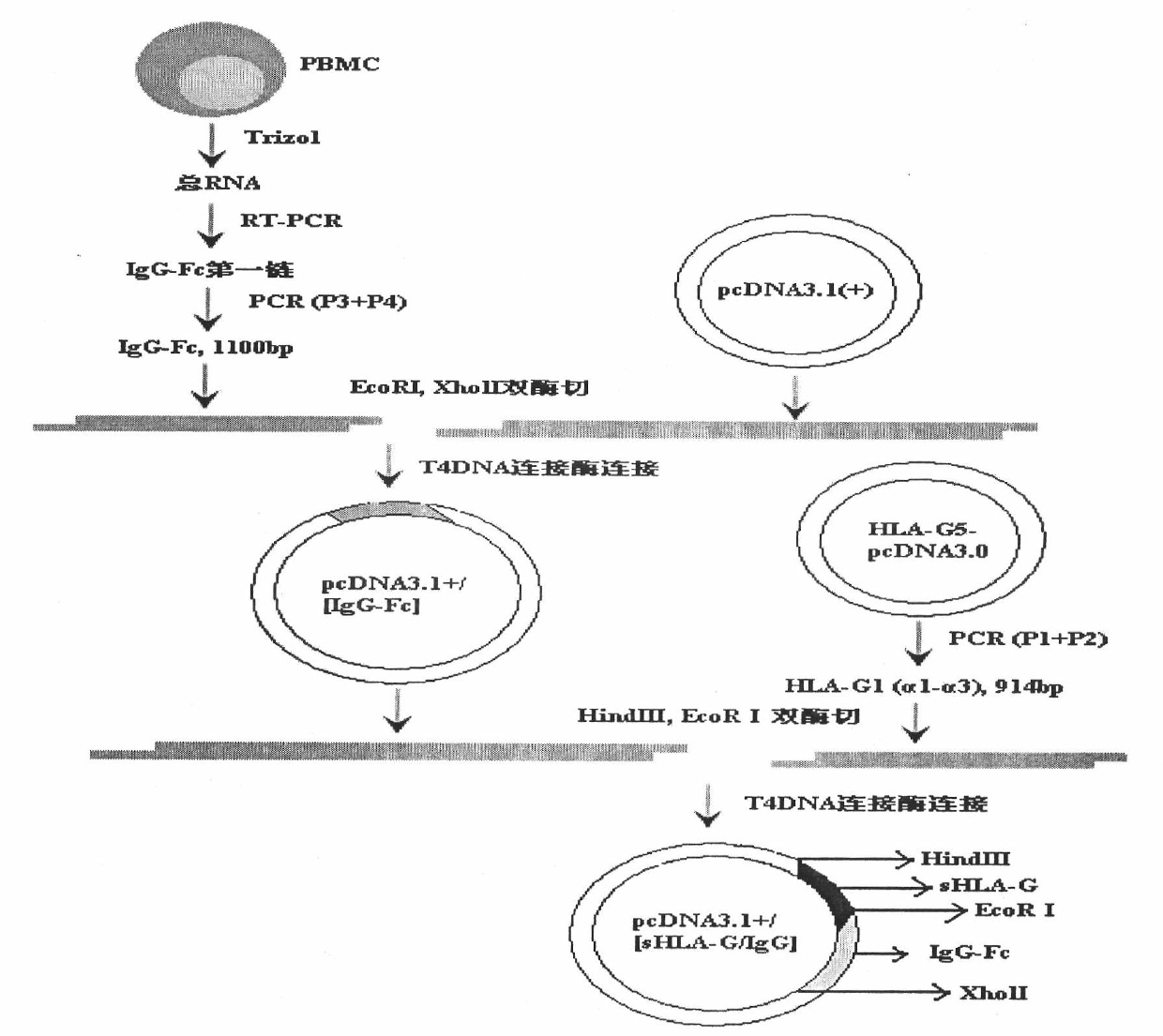
[0060] Select the HLA-G5-pcDNA3.0 plasmid and normal human peripheral blood cells to obtain the sHLA-G heavy chain extracellular segment α1-α3 region gene and the human IgG1 heavy chain constant region (IgG-Fc segment) encoding gene, and IgG-Fc Insert the gene fragment of the eukaryotic expression plasmid pcDNA3.1+ to form pcDNA3.1+ / [IgG-Fc], and then recombine the sHLA-G extracellular segment gene into pcDNA3.1+ / [IgG-Fc] to construct a pcDNA3.1+ / [sHLA-G / IgG] plasmid of fusion gene, see the technical route figure 1 .
[0061] 1.1 Obtain the gene fragments of the fusion protein sHLA-G / IgG
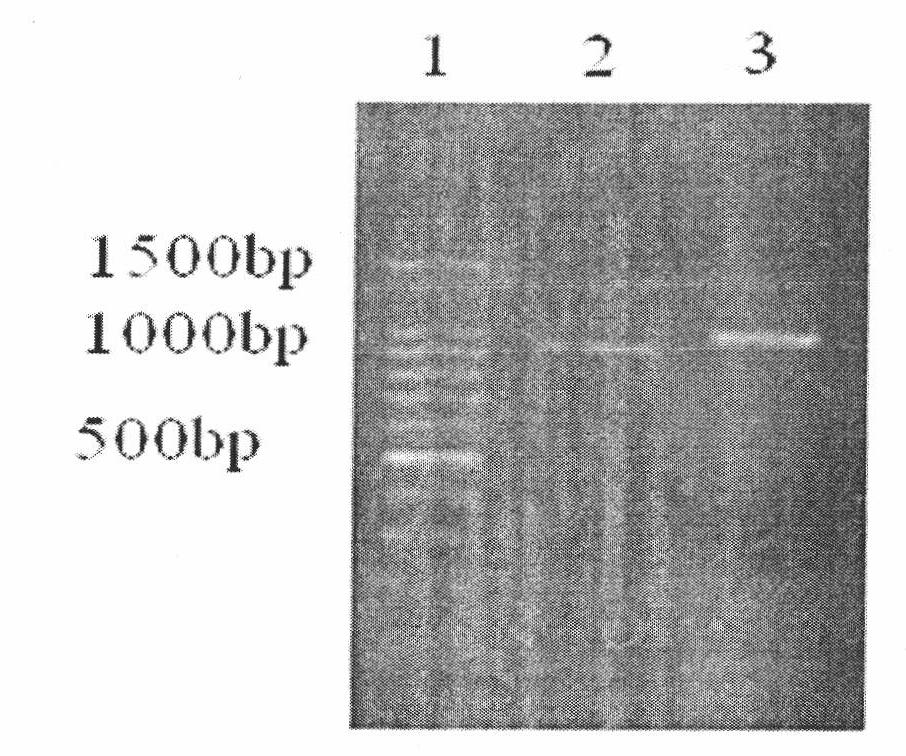
[0062] The gene of the recombinant sHLA-G / IgG fusion protein is formed by fusing the extracellular segment (α1-α3 region) of HLA-G1 with the encoding gene of the CH1-CH3 segment of IgG1. The IgG-Fc segment gene amplified by RT-PCR method includes the CH1-CH3 region coding gene of IgG1 and res...
Embodiment 2
[0067] Example 2 Expression, purification and identification of fusion protein sHLA-G / IgG
[0068] 2.1 Establishment of cell lines expressing fusion protein sHLA-G / IgG
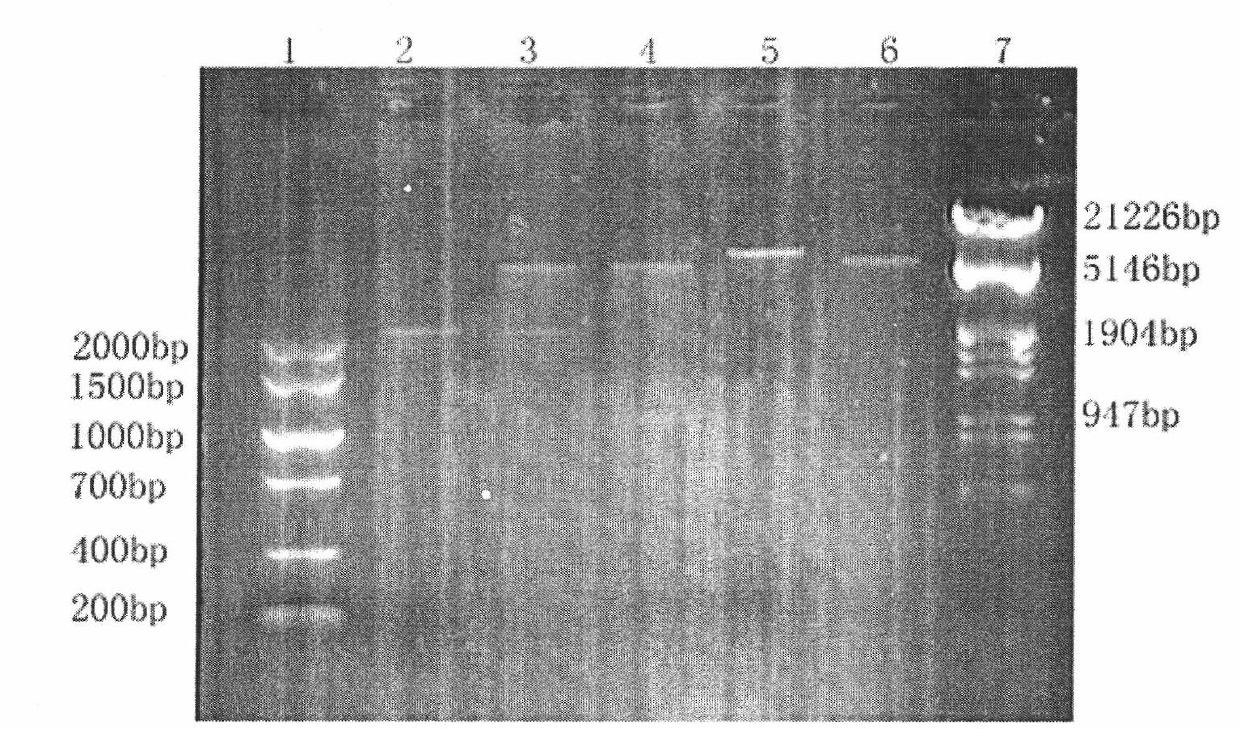
[0069] In order to obtain higher protein expression and exclude the interference of MHC molecules and IgG expressed by the cells themselves, we chose LCL 721.221 cells as the expression cells, which are defective in the expression of HLA class I molecule heavy chain and IgG1 heavy chain [30] , but high expression of HLA class I light chain (β 2 M). The plasmid pcDNA3.1+[sHLA-G / IgG] was transfected into 721.221 cells by electroporation, and selected using G418-containing selective medium. After the stable growth of cell clones in G418 was obtained, monoclonalization was carried out, and the expression level of HLA-G fusion protein was further detected, and the clone with efficient and stable expression of the target protein was selected as dimer-721.221, and a large number of cells were expanded and preserved...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com