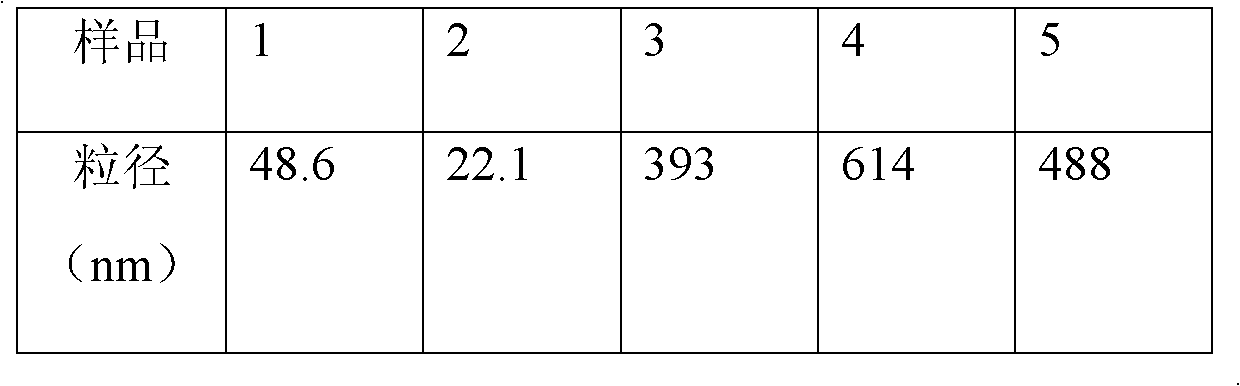Medicinal composition of 24-methylene cycloartenyl ferulate
A technology of pineapple ferulate and methylene cycloartide, which is applied in the field of medicine and can solve the problems of insoluble solubilization and low solubility of 24-methylene cycloartenol ferulate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
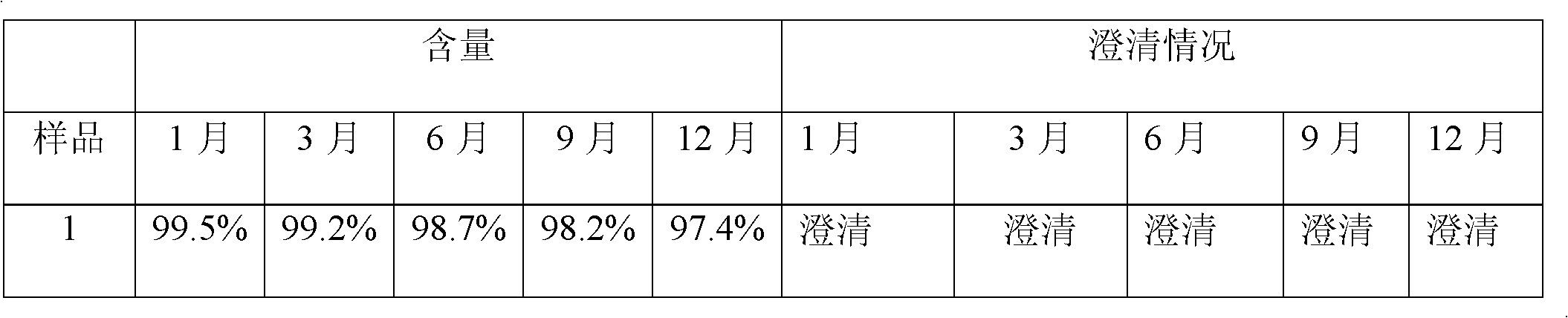
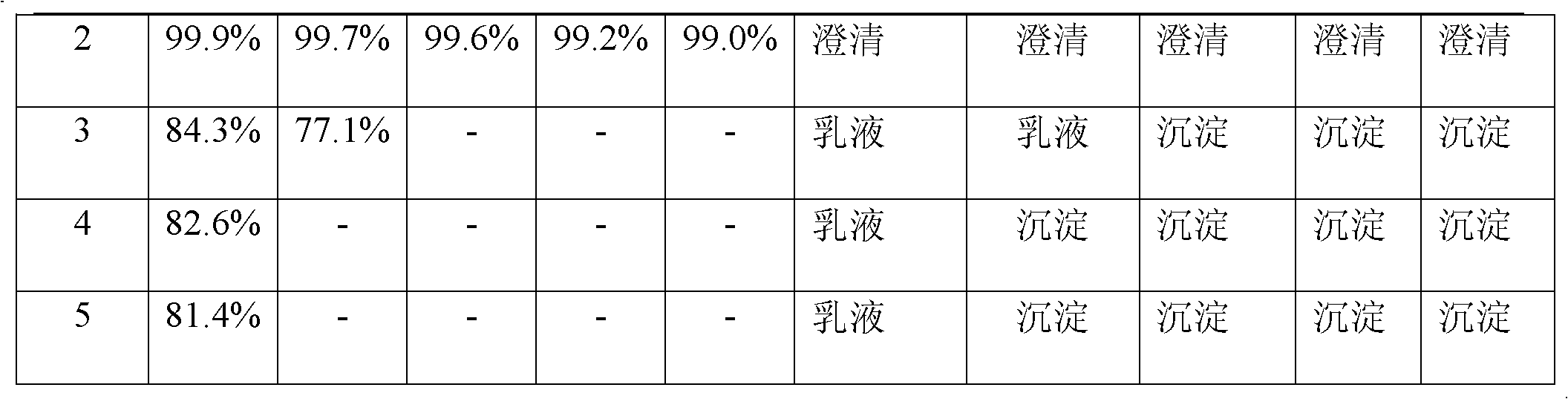
[0028] Take 20g of s40, heat it to a molten state at 60°C, then add 4g of 24-methylene cycloartenyl ferulic acid ester to it, mix and stir for 30min, add the resulting molten liquid into 5L of water for injection and stir until completely dissolved , the obtained pharmaceutical composition of the present invention, the concentration is 0.8mg / ml.
[0029] The resulting solution cleared completely on standing at room temperature for 24 hours.
[0030] The obtained pharmaceutical composition solution is filtered with a 0.45um filter membrane, and after passing the test, it is packed into an oral liquid bottle to obtain 400 oral preparations of the pharmaceutical composition of the present invention.
Embodiment 2
[0032]Take 100g of s32, heat it to a molten state at 60°C, then add 5g of 24-methylene cycloartenyl ferulic acid ester to it, mix and stir for 50min, add the obtained molten liquid into 3L of water for injection and stir until completely dissolved , the obtained pharmaceutical composition of the present invention, the concentration is 0.7mg / ml.
[0033] The resulting solution cleared completely on standing at room temperature for 24 hours.
[0034] The obtained pharmaceutical composition solution was stirred with 0.3% active carbon for injection for 30 minutes, decarbonized and filtered, and finely filtered through a 0.22um microporous filter membrane, then packed into vials to obtain 500 injections of the pharmaceutical composition of the present invention.
Embodiment 3
[0036] Take 200g of s40, heat it to a molten state at 70°C, then add 4g of 24-methylene cycloartenyl ferulic acid ester to it, mix and stir for 30min, add the resulting molten liquid to 4L of water for injection and stir until completely Dissolved to obtain the pharmaceutical composition of the present invention with a concentration of 1 mg / ml.
[0037] The resulting solution cleared completely on standing at room temperature for 24 hours.
[0038] The above pharmaceutical composition solution was added with 0.3% activated carbon for injection and stirred for 30 minutes. After decarburization and filtration, after fine filtration with a 0.22um microporous membrane, the following freeze-drying process was adopted:
[0039] Pre-freezing: The temperature of the product drops to -45°C, and it can be sublimated and dried after 3 hours of heat preservation;
[0040] Sublimation drying: the sublimation drying temperature is controlled below -12°C;
[0041] Re-drying: the maximum te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com