Rebeccamycin analogue with anticancer activity and synthesis method thereof
A technique for phathomycin and anticancer activity, which is applied in the field of phathomycin analogs with anticancer activity and the field of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
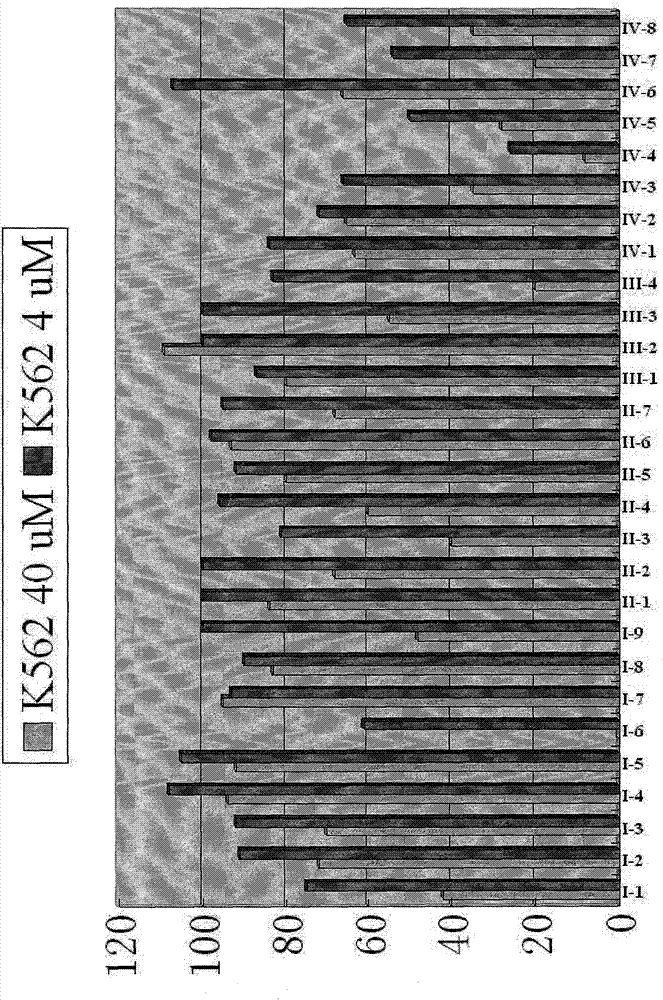
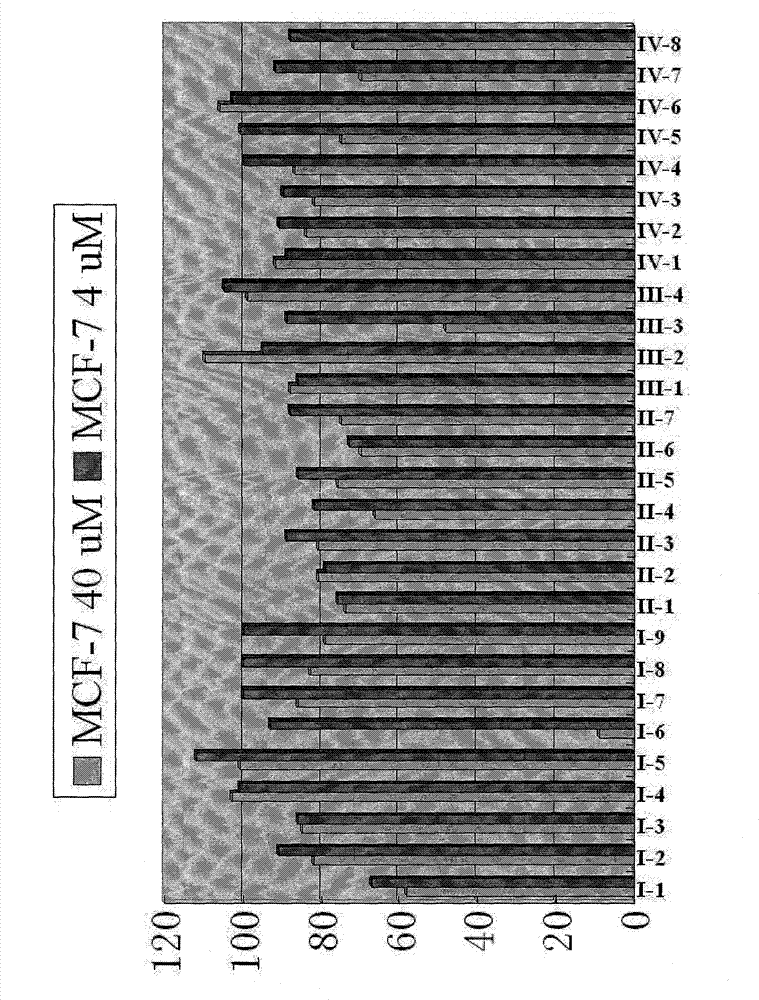
[0027] 1. Synthesis of N-glycosyl phaeomycin analog I series compounds
[0028] We choose the following six glycosyl intermediates as examples to illustrate the synthesis of N-glycosyl phaeomycin analog I series compounds.
[0029]
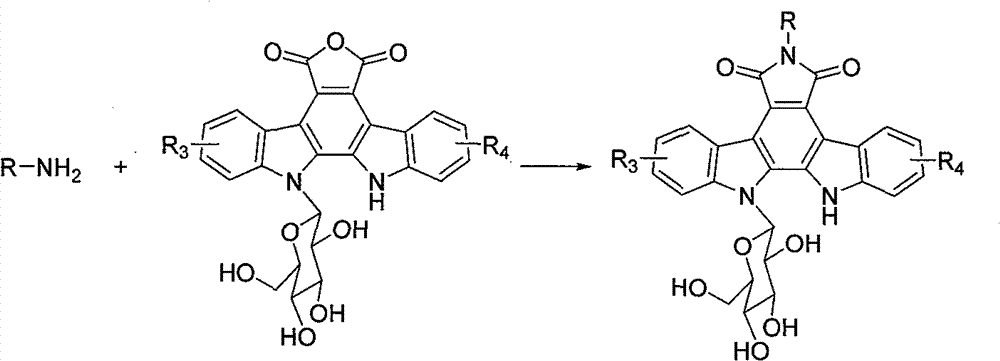
[0030] Starting from various sugars and aminoethanol, we synthesized six kinds of glycosyl intermediates according to the following synthetic route, and chose the following glycosyl intermediates as an example to illustrate the synthesis of Phetalemycin analogues:
[0031]
[0032] 1) Synthesis of N-Phth aminoethanol
[0033] Add phthalic anhydride (1mol) into a 10mL round-bottomed flask, and then add aminoethanol (1eq) dropwise into the flask with stirring, and the reaction is exothermic. After the dropwise addition, the temperature was raised to 100° C. with an oil bath, and the reaction was completed after about 1 hour. The temperature of the solution was lowered to room temperature, and the solution became solid. Distilled water was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com