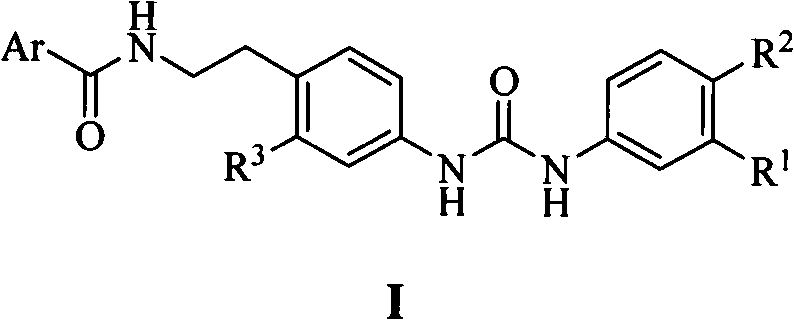
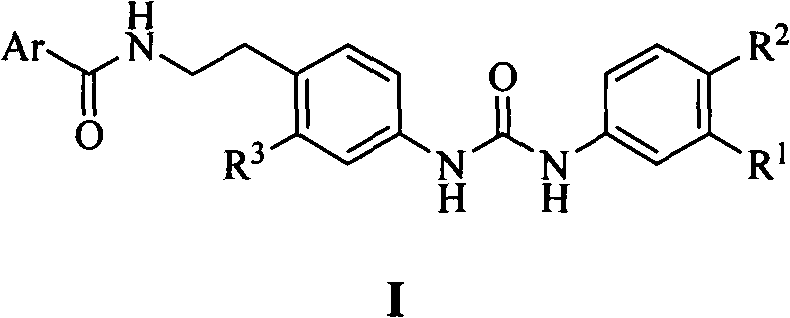
N,N'-bis-substituted urea Raf kinase inhibitors and preparation method and application thereof
A C1-C6 compound technology, applied in the field of medicinal chemistry, can solve problems such as limited therapeutic range and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
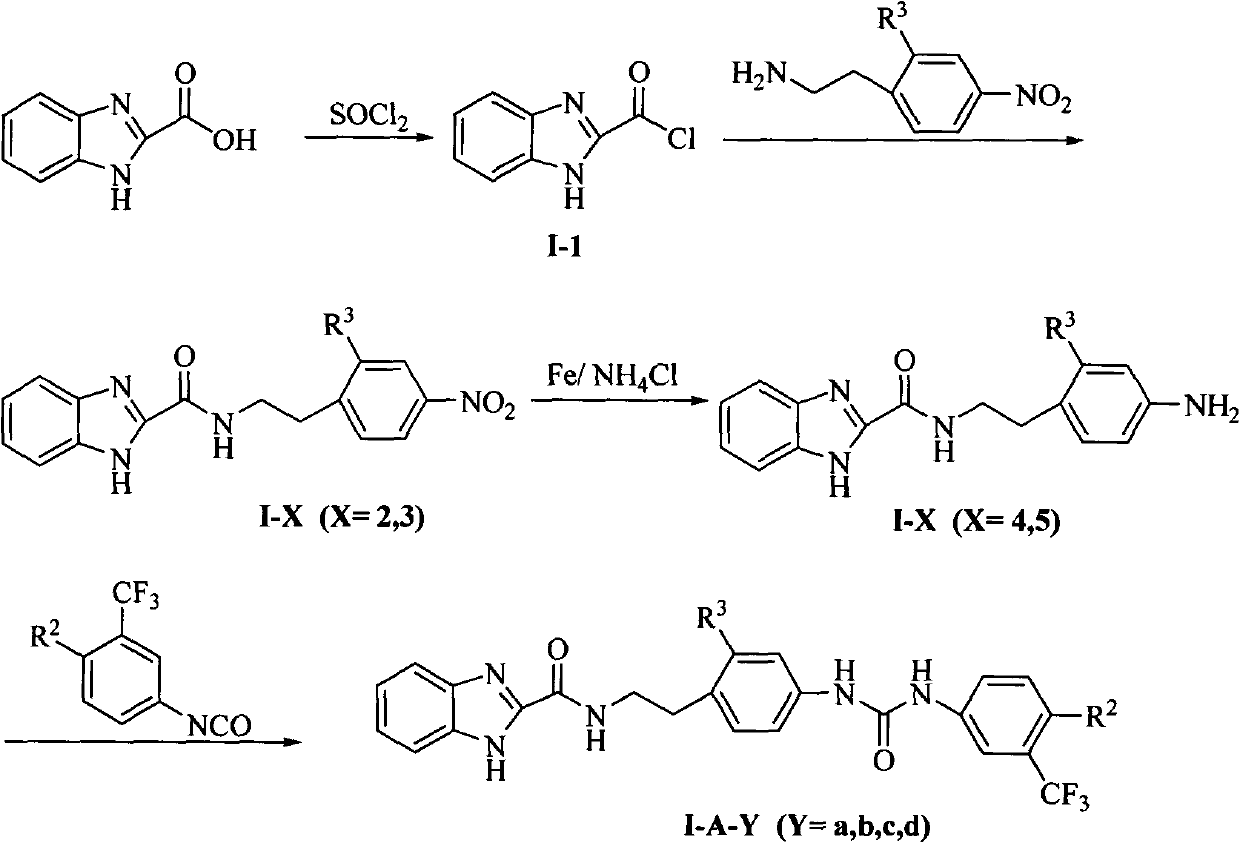
[0087] 1H-Benzo[d]imidazole-2-carbonyl chloride (I-1)
[0088] Add 8.1 g (50 mmol) of 1H-benzo[d]imidazole-2-carboxylic acid and 40 mL of thionyl chloride into a 150 mL single-necked bottle, and heat to reflux for 4 hrs. The thionyl chloride was distilled off under reduced pressure to obtain 7.7 g of yellow solid (I-1) with a yield of 86.0%. The product did not need further purification and was directly used for the next reaction.
Embodiment 2
[0090] N-[2-(4-nitrophenyl)ethyl]-1H-benzo[d]imidazole-2-carboxamide (I-2)
[0091] Add 4-nitrophenethylamine 9.6g (58mmol), anhydrous chloroform 50mL and anhydrous triethylamine 5mL in 250mL three-necked bottle, slowly drop the chloroform suspension of 1-17.0g (38.7mmol) at room temperature ( 25 mL), heated to reflux for 4 hr after the addition, and TLC detected that the starting material disappeared (methanol:chloroform=1:30). Chloroform was distilled off, about 50 mL of water was added, stirred, allowed to stand, and filtered with suction to obtain 9.94 g of yellow solid (I-2), with a yield of 83.1%. The product did not need further purification and was directly used in the next reaction. 1 H-NMR [300MHz, DMSO-d 6 ]: δ13.16(1H, s, benzimidazole-NH); 9.04(1H, t, CO-NH); 8.13(2H, dd, ArH); 7.69(1H, m, ArH); 7.56(1H, m, ArH); 7.53 (2H, dd, ArH); 7.28 (2H, m, ArH); 83.61 (2H, q, CH 2 ); δ3.05(2H, t, CH 2 ).
Embodiment 3
[0093] N-[2-(4-aminophenyl)ethyl]-1H-benzo[d]imidazole-2-carboxamide (I-4)
[0094] Add I-29g (29mmol), iron powder 6.5g (116mmol), NH 4 Cl 3.1g (58mmol) and 75% ethanol (100mL) were heated to reflux for 5hrs, and the starting material disappeared as detected by TLC (methanol:chloroform=1:30). Suction filtration while hot, and the filter cake was washed with a small amount of ethanol. Most of the solvent was evaporated under reduced pressure, extracted three times with ethyl acetate (20mL×3), the combined extracts were washed once with water and saturated sodium chloride respectively (20mL×1), the solvent was evaporated under reduced pressure to obtain a yellow oil, The crude product was subjected to column chromatography (developing solvent: methanol: chloroform = 1:30) to obtain I-45.74g with a yield of 70.6%. 1 H-NMR [300MHz, DMSO-d 6 ]: δ13.16 (1H, s, benzimidazole); 8.82 (1H, t, CO-NH); 7.69 (1H, m, ArH); 7.53 (1H, m, ArH); 7.28 (2H, m, ArH) ; 6.88 (2H, dd, ArH); 6.74...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com