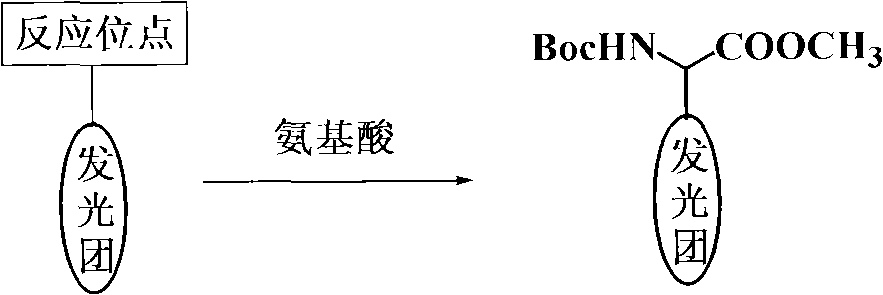
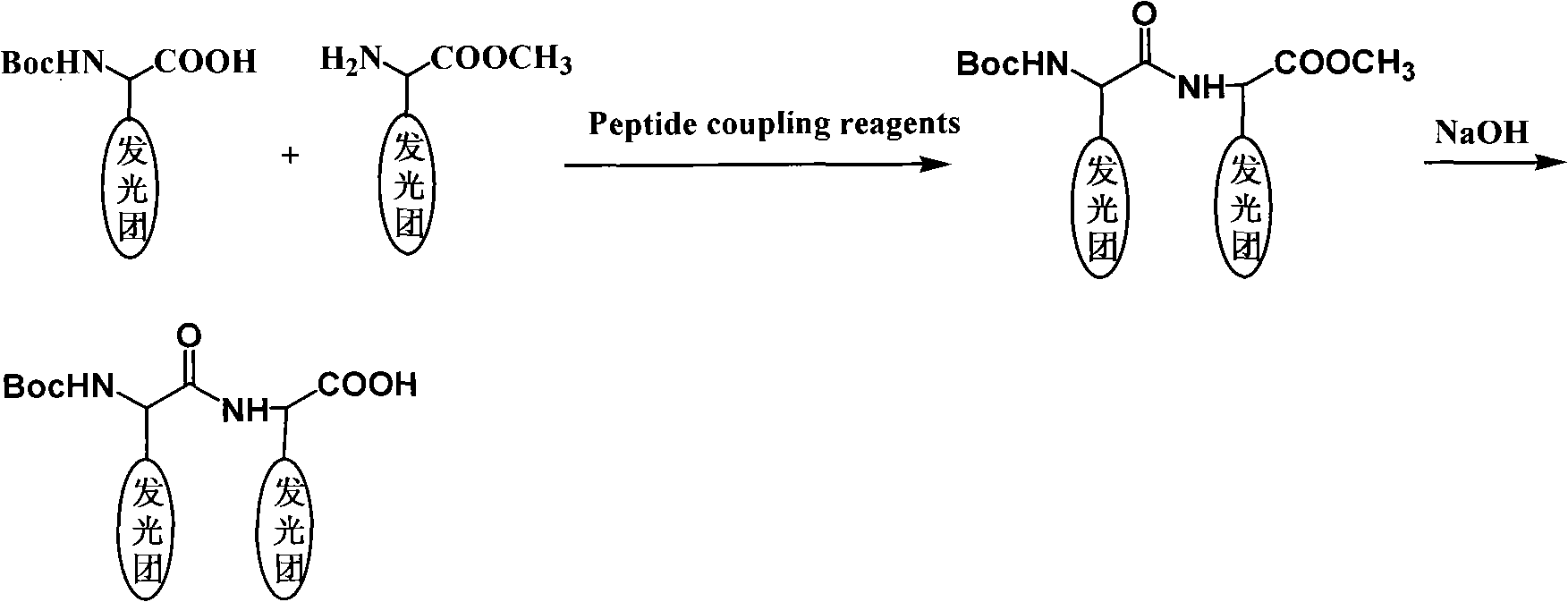
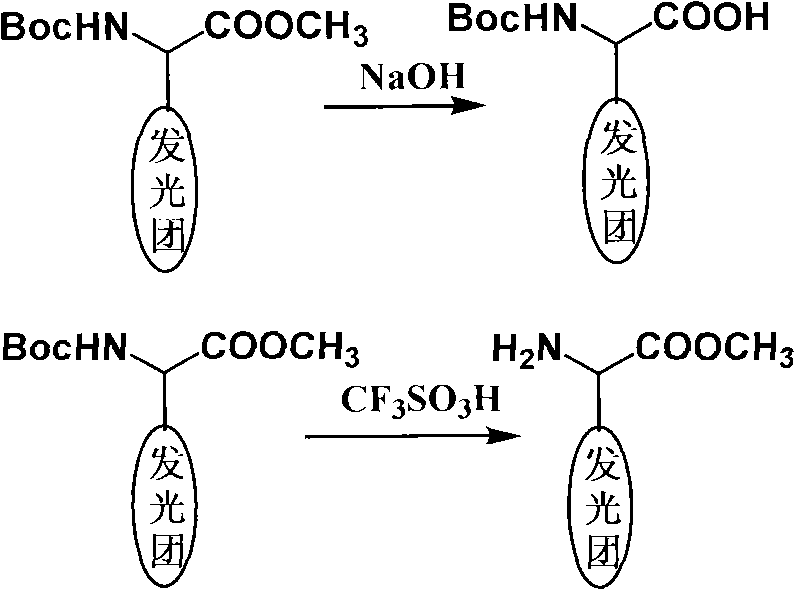Coding molecule for marking biomolecule and preparing method thereof
A technology of biomolecules and encoding, which is applied in the field of encoding molecules for labeling biomolecules and its preparation, and can solve problems such as narrow emission peaks, different, and poor photochemical stability of organic fluorescent substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of encoding molecules whose backbone is linear
[0034]
[0035] The synthetic steps of compound 3: get compound 1 (1mmol), HOSu (1mmol) in a 50 milliliter flask, add 20 milliliters of dry DMF, add DCC (1mmol), stir at room temperature for 20 hours, add Boc-lysine (2mmol ), DIEA (1mmol), stirred at room temperature for 24 hours, the reaction was completed, the solvent was spun off, and the column was passed to obtain the product. Elemental analysis: C, 49.42%; H, 3.82%; N, 7.04% (found value); C, 49.39%; H, 3.8 / 4%; N, 7.02% (C 41 h 38 f 4 IrN 5 o 8 theoretical value).
[0036] The synthesis steps of compound 4: take compound 2 (1mmol), Boc-lysine methyl ester (2mmol), DMAP (1mmol) in a 100 ml flask, add 20 ml of toluene, add DCC (1mmol), stir at room temperature for 2 hours After the reaction was completed, the solvent was spun off, 60 milliliters of dichloromethane was added, trifluoroacetic acid (6 mmol) was added, and stirred at room tem...
Embodiment 2
[0038] Example 2: Synthesis of Encoding Molecules with Dendritic Main Backbone
[0039]
[0040] Compound 10
[0041] The synthetic steps of compound 9: get compound 6 (1mmol), DMAP (1mmol) in a 100 milliliter flask, add 20 milliliters of DMF, get compound 8 (1mmol) and make DMF solution, under magnetic stirring, the solution that is made is dripped Add it to the reaction bottle, after the dropwise addition is completed, stir at room temperature for 24 hours. After the reaction is complete, spin off the solvent and pass through the column to obtain the product. Elemental analysis: C, 63.86%; H, 4.95%; N, 6.05% (found value); C, 63.84%; H, 4.92%; N, 6.08% (C 49 h 45 EuN 4 o 5 theoretical value).
[0042] The synthetic steps of compound 10: take compound 7 (1mmol) in a 100 milliliter flask, add 20 milliliters of DMA, take compound 9 (1mmol) and add 10 milliliters of DMA to form a solution, and add the solution dropwise to the reaction flask under stirring , the dropwise a...
Embodiment 3
[0043] Example 3 Compound 5 labels BSA (bovine serum albumin) under the effects of EDC and NHS
[0044] NHS (1.05 mmol) and EDC (1 mmol) were added to compound 5 (1 mmol) in PBS buffer solution (pH=7.4), reacted at room temperature for 30 minutes, then added BSA, and stirred overnight at room temperature. The resulting mixture was purified by dialysis in PBS solution with a molecular weight cut-off of 10000 Da for three days to remove uncrosslinked compounds, that is, to obtain BSA crosslinked with the labeled compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com