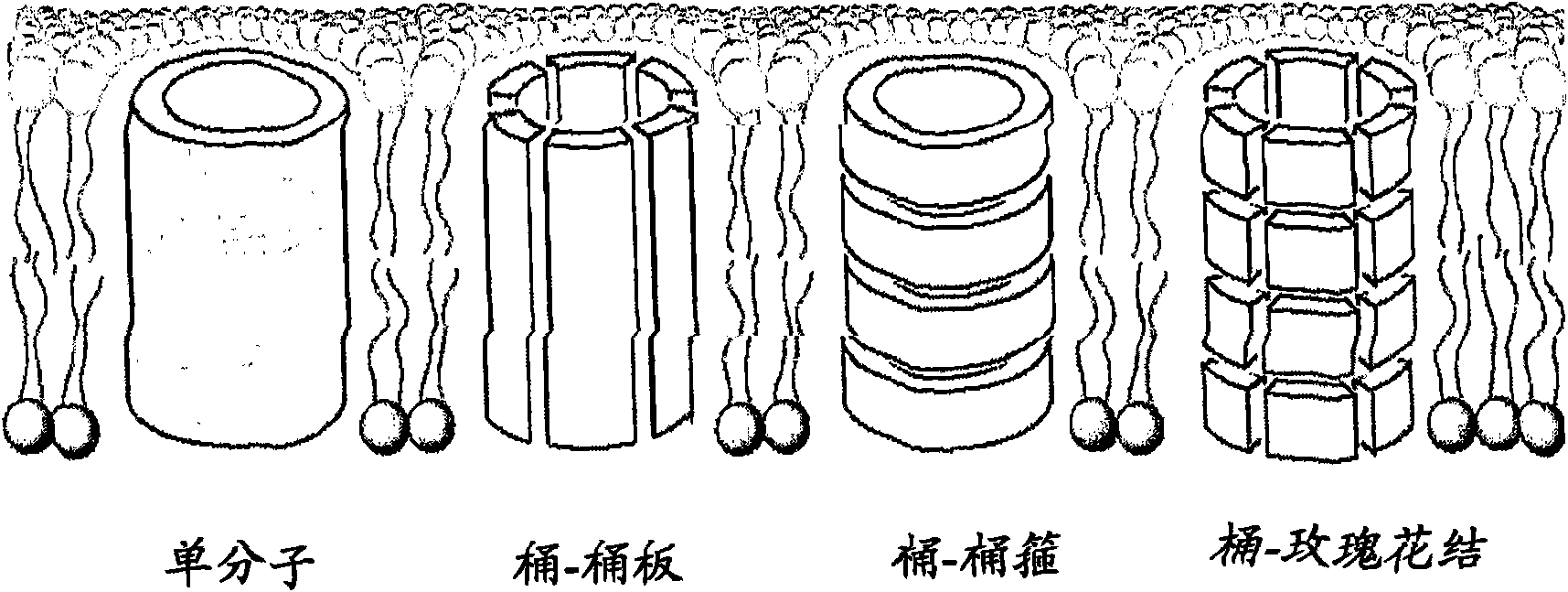
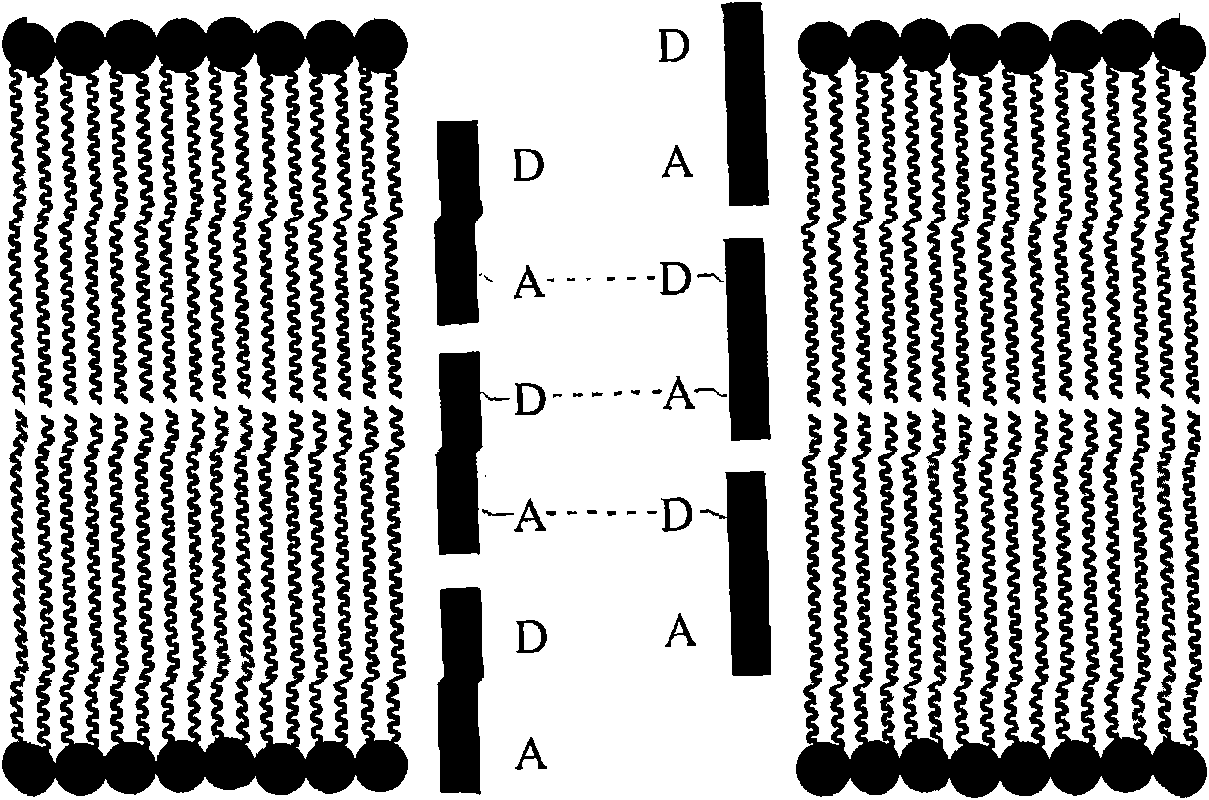
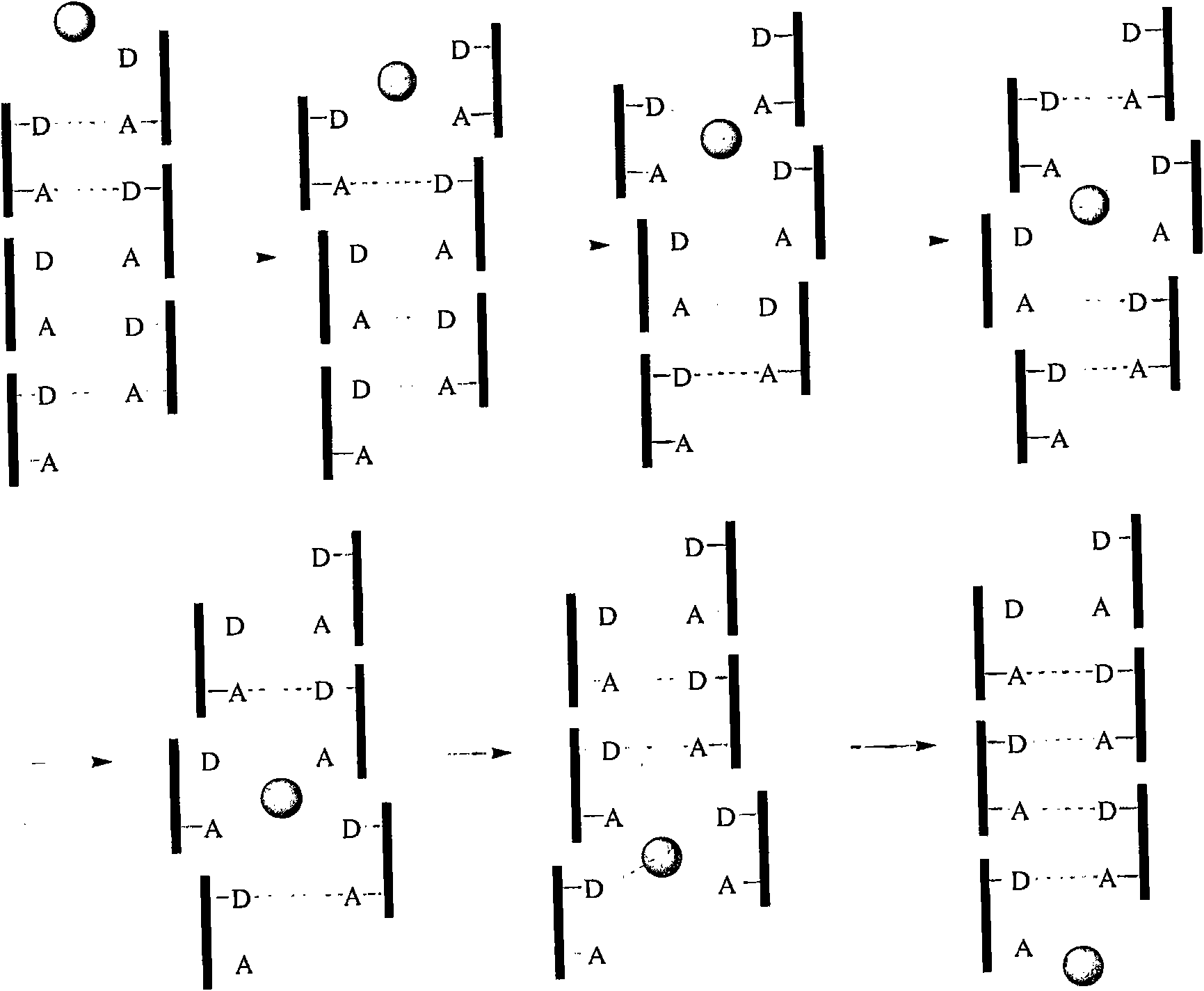
Method of modulating membrane potential of a cell
A technology of cell membrane and membrane potential, applied in pharmaceutical formulations, medical preparations containing active ingredients, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Preparation of Example 1
[0205]
[0206] Process A
[0207] Example 1 was prepared according to Scheme A above. According to Yang et al., J.Org.Chem, 2001, 66, 7303-7312 described procedure synthesis starting material 2-phthalimidoxy (phthalimidoxy)-4-methylpentanoic acid D-tert-butyl ester ( Compound 1). Compound 1 is a white crystalline solid characterized by the following data: melting point 92-93 °C; [α] 20 D +77.0° (c 1.01, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ7.85-7.81 (m, 2H), 7.78-7.74 (m, 2H), 4.74 (dd, J=8.5, 5.4Hz, 1H), 2.05-1.91 (m, 2H), 1.72-1.63 (m, 1H), 1.46(s, 9H), 1.07(d, J=6.3Hz, 3H), 1.00(d, J=6.3Hz, 3H); 13 C NMR (75MHz, CDCl 3 )δ169.13, 163.21, 134.50, 128.87, 123.53, 84.74, 83.39, 39.89, 27.82, 24.47, 22.90, 21.96; IR (CHCl 3 )3032, 1793, 1738cm -1 ; LRMS (EI, 70ev) m / z 333 (M + , 1), 278(6), 232(17), 164(15), 148(100); C 18 h 23 NO 5 (M + ) HRMS (EI): theoretical value 333.1576, measured value 333.1573.
[0208] To the CH of c...
Embodiment 2
[0210] Preparation of Example 2
[0211]
[0212] Process B
[0213] Example 2 was prepared according to Scheme B above. CH 2 Cl 2 (5mL) solution was carefully added an equal volume of CF 3 COOH (5 mL). After stirring at room temperature for 3 hours, the reaction mixture was concentrated in vacuo. The residue was azeotroped twice with toluene to give the free acid compound 3 as a white solid which was used directly for peptide coupling.
[0214] Freshly distilled CH 2 Cl 2 (50 mL) was added to the flask containing dry free acid compound 3, followed by HOAt (354 mg, 2.6 mmol), isobutylamine (0.21 mL, 2.1 mmol) and finally EDCI (891 mg, 3.0 mmol). After overnight stirring, CH 2 Cl 2 Dilute the reaction mixture. Organic layer with 5% NaHCO 3 and brine, followed by anhydrous MgSO 4 Dried and concentrated. The crude oil was purified by flash column chromatography to afford 492 mg of Example 2 (92% yield) as a white solid. Example 2 is characterized by the followin...
Embodiment 3
[0215] Preparation of Example 3
[0216]
[0217] Process C
[0218] Example 3 was prepared following Scheme C above similar to Scheme B for Example 2, except that decylamine was substituted for isobutylamine. Example 3 isolated as a colorless oil. Example 3 is characterized by the following data: [α] 20 D +34.5° (c 1.00, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ11.32(s, 2H), 8.28(br, 2H), 8.19(s, 1H), 8.05(d, J=7.5Hz, 2H), 7.52(t, J=7.5Hz, 1H), 4.38( br, 2H), 3.13-3.02(m, 4H), 1.82(m, 2H), 1.65-1.55(m, 4H), 1.42(br, 4H), 1.35-1.25(m, 28H), 0.89-0.85( m,18H); 13 C NMR (100MHz, CDCl 3 )δ172.30, 165.91, 132.05, 131.29, 129.37, 124.98, 85.31, 41.17, 39.47, 31.89, 29.55, 29.53, 29.30, 29.12, 26.91, 24.73, 23.18, 22.67, 21.79; IR, 14.Cl 3 )3446, 1662cm -1 ; LRMS (FAB) m / z 704 (M + , 1); C 40 h 71 N 4 o 6 (M + , 1) HRMS (FAB): theoretical value 703.5374, measured value 703.5354.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com