Antimicrobial gel formulations
A preparation and derivative technology, applied in the field of stable antimicrobial preparations, can solve the problems of unstable compounds, difficult compound preparation, etc., and achieve the effect of increasing effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
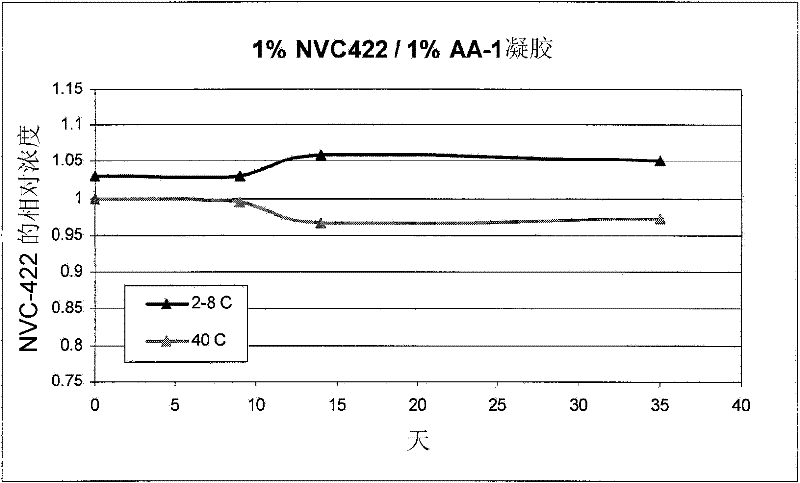
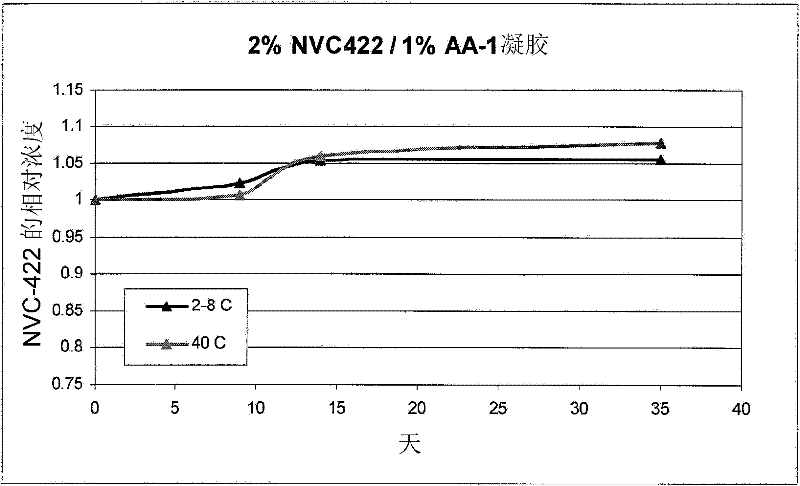
[0272] Example 1: AA-1 Gel Formulation
[0273] A formulation of 1% N,N-dichloro-2,2-dimethyltaurine in 1% AA-1 was prepared by the following method. Prepared by slowly adding the gelling agent to the water while stirring to prevent the gelling agent from clumping 1.5% (w / v) solution of AA-1 polycarbophil. A 4% (w / v) solution of N,N-dichloro-2,2-dimethyltaurine was prepared separately. The above two solutions were mixed to form a 1% N,N-dichloro-2,2-dimethyltaurine / 1% AA-1 solution. The pH of the solution was adjusted to about 5.0 with HCl (NaOH if necessary).
Embodiment 2
[0274] Embodiment 2: Eucalyptol preparation
[0275] A solution of 1% N,N-dichloro-2,2-dimethyltaurine ("NVC-422") in 0.2% cineole was prepared by mixing 1% (w / v) NVC-422 with 100ml 0.9% salt (NaCl) solution was mixed, and then 0.2% 1,8-cineole (v / v) was added. The resulting solution was clear and colorless, and had a pungent, eucalyptus-like odor.
Embodiment 3
[0276] Embodiment 3: 3-octanone preparation
[0277] A 1% NVC-422 solution in 0.5% 3-octanone was prepared by mixing 1% NVC-422 (w / v) with 100 ml of a 0.9% salt (NaCl) solution and then adding 0.5% 3-octyl Ketones (v / v). The resulting solution was clear and colorless and had a strong, sweet, floral odour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com