Process for production of optically active organic carboxylic acid
An organic carboxylic acid, optically active technology, applied in organic chemical methods, preparation of organic compounds, preparation of carboxylates, etc., can solve the problems of low enantiomeric excess rate, time-consuming and labor-intensive, etc. Effect of Conform Excess Rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
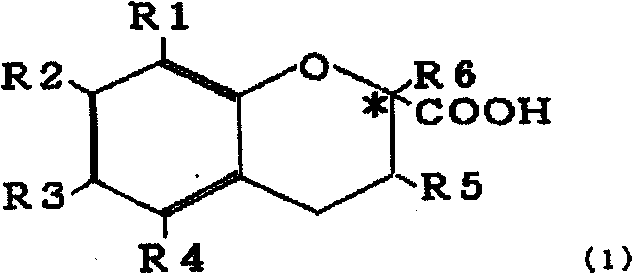
[0065] Asymmetric Esterification of 6-Hydroxy-2,5,7,8-Tetramethylchroman-2-Carboxylic Acid
[0066] 100 g (399.5 mmol) of raw materials 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid, 60 g of methanol, and 1,500 g of isopropyl ether were added to a 5 L stainless steel pressure vessel. 40 g of immobilized enzyme Novozym 435 (manufactured by Novozymes) as a symmetric esterification catalyst was replaced with argon, and reacted at 80° C. for 24 hours. After 24 hours, the catalyst was removed by filtration, and the reaction solution was recovered. Analyzing the reaction solution, the transformation rate of raw material 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid is 45.0mol%, S-(-)-6-hydroxyl-2,5,7 , The optical purity of methyl 8-tetramethylchroman-2-carboxylate was 99% ee. On the other hand, the optical purity of R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid was 81%ee. In addition, the analysis of optical purity was performed by high perform...
Embodiment 1
[0068](1) recovery of unreacted 6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid
[0069] Add 219.7ml (399.5mmol÷10×(1-0.45)=21.97mmol) of 0.1N sodium hydroxide to 10 aliquots of the above-mentioned reaction solution to make unreacted 6-hydroxyl-2,5,7,8-tetra Methylchroman-2-carboxylic acid was removed as a sodium salt and recovered as an aqueous layer.
[0070] (2) Recovery of R-(+)-6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid
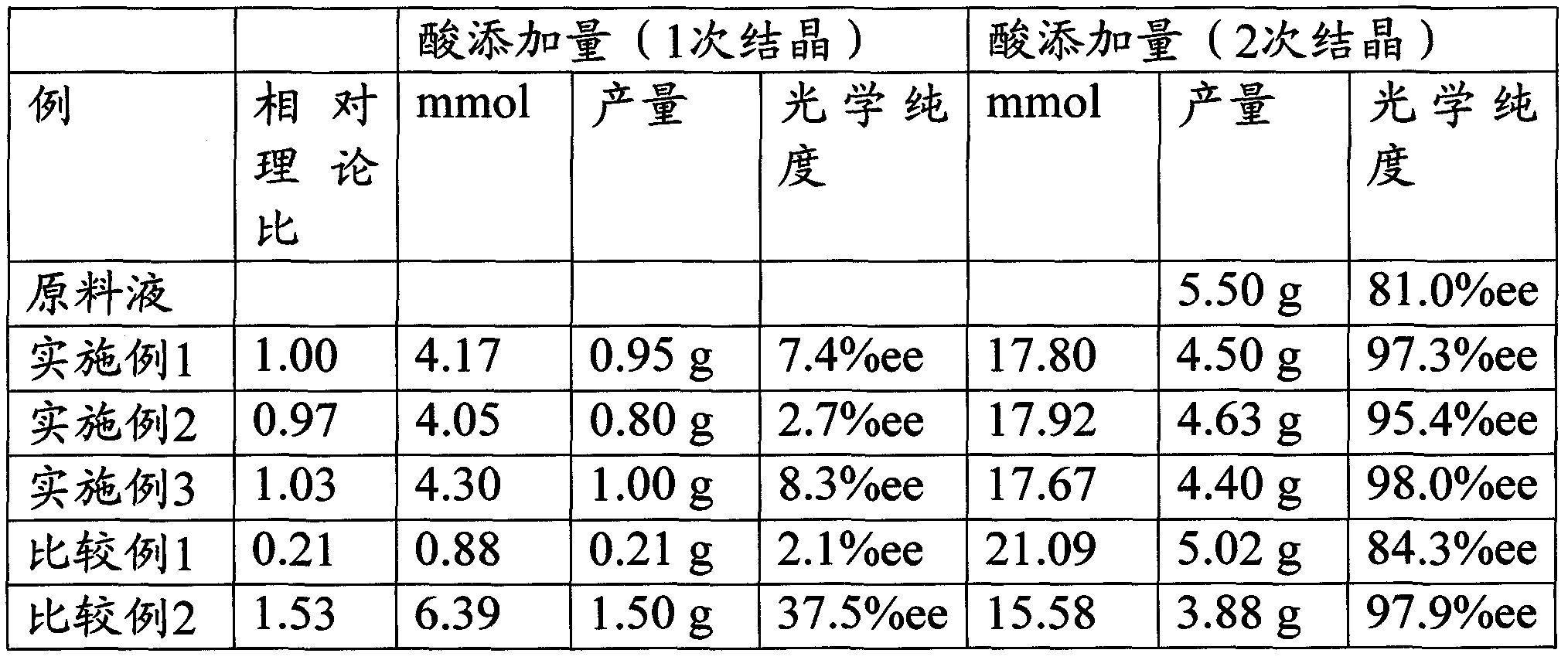
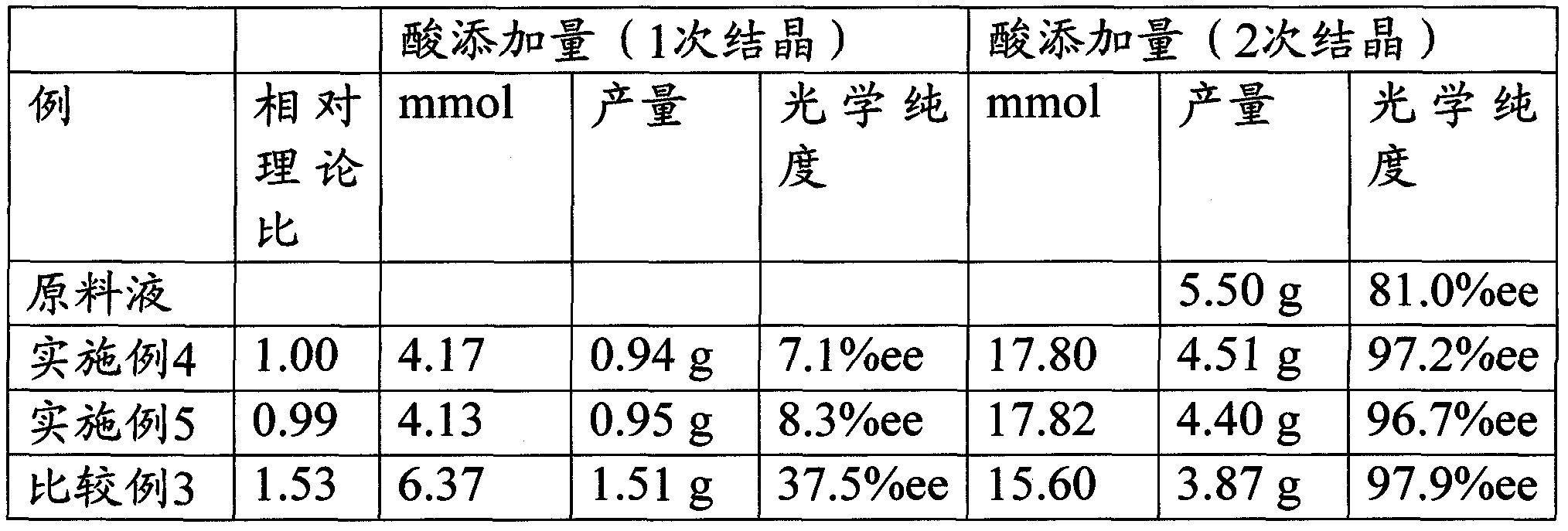
[0071] The water layer recovered in the above (1) was added to a 500mL glass container, and while heating and stirring at 50°C, 4.17ml (21.97mmol×(100- 81) / 100=4.17mmol), and the free primary crystal was filtered out. Next, the remaining 17.80 ml of 1N hydrochloric acid was added to the obtained filtrate, and free secondary crystals were filtered off.
[0072] The yield of one crystallization was 0.95 g, and the optical purity of R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid in the crystal was 7.4%ee.
[0073] The ...
Embodiment 2
[0075] The water layer recovered in (1) of Example 1 was added to a 500mL glass container, and while heating and stirring at 50°C, 4.05ml (21.97ml× (100-81) / 100×0.97 times), the free primary crystals were filtered out. Next, the remaining 17.92 ml of 1N hydrochloric acid was added to the obtained filtrate, and free secondary crystals were filtered off.
[0076] The yield of one crystallization was 0.80 g, and the optical purity of R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid in the crystal was 2.7%ee.
[0077] The yield of the second crystallization was 4.63 g, and the optical purity of R-(+)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid in the crystal was 95.4%ee. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com