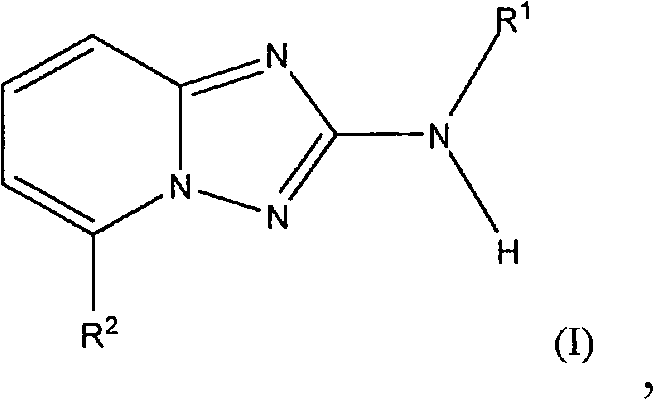
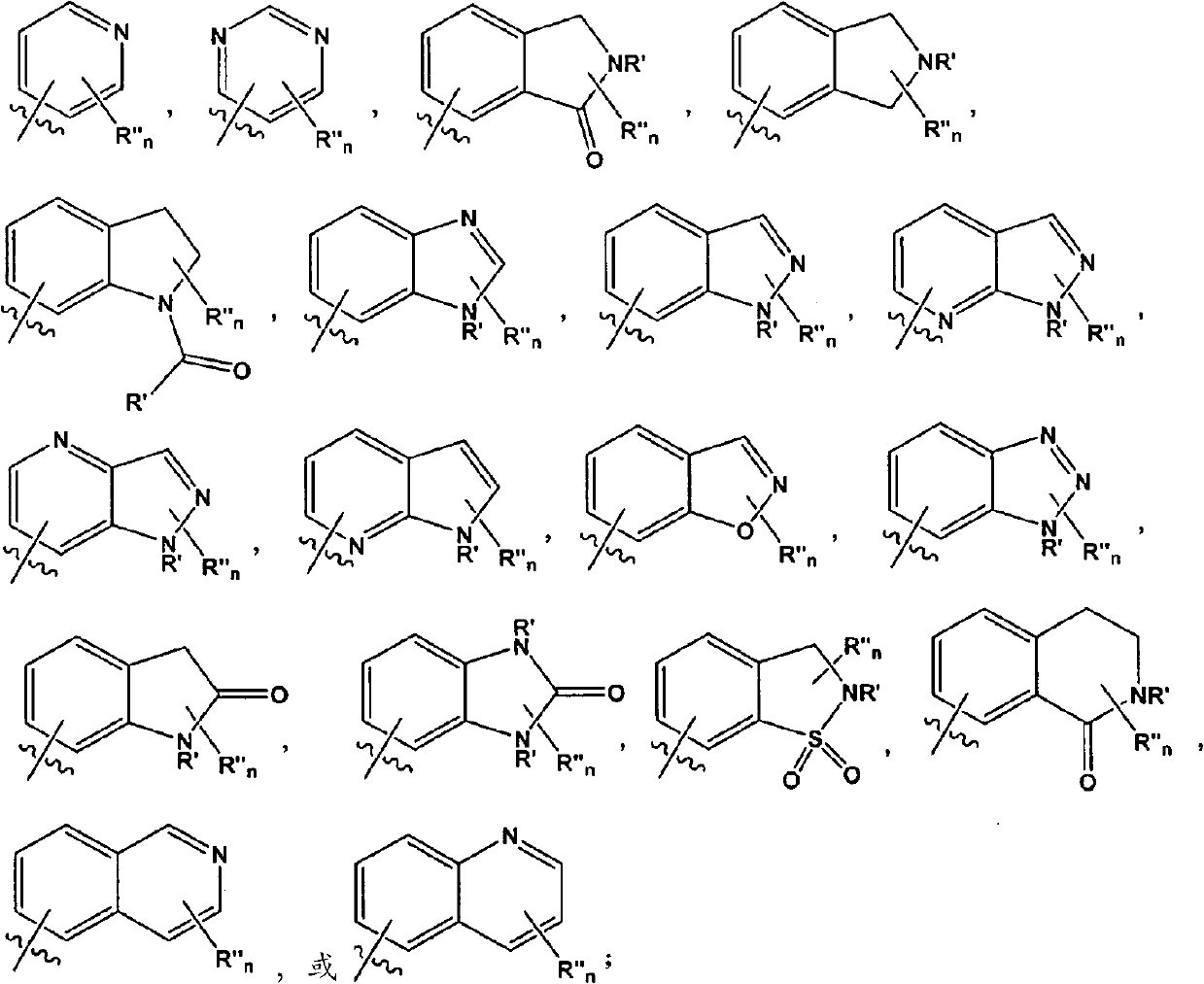
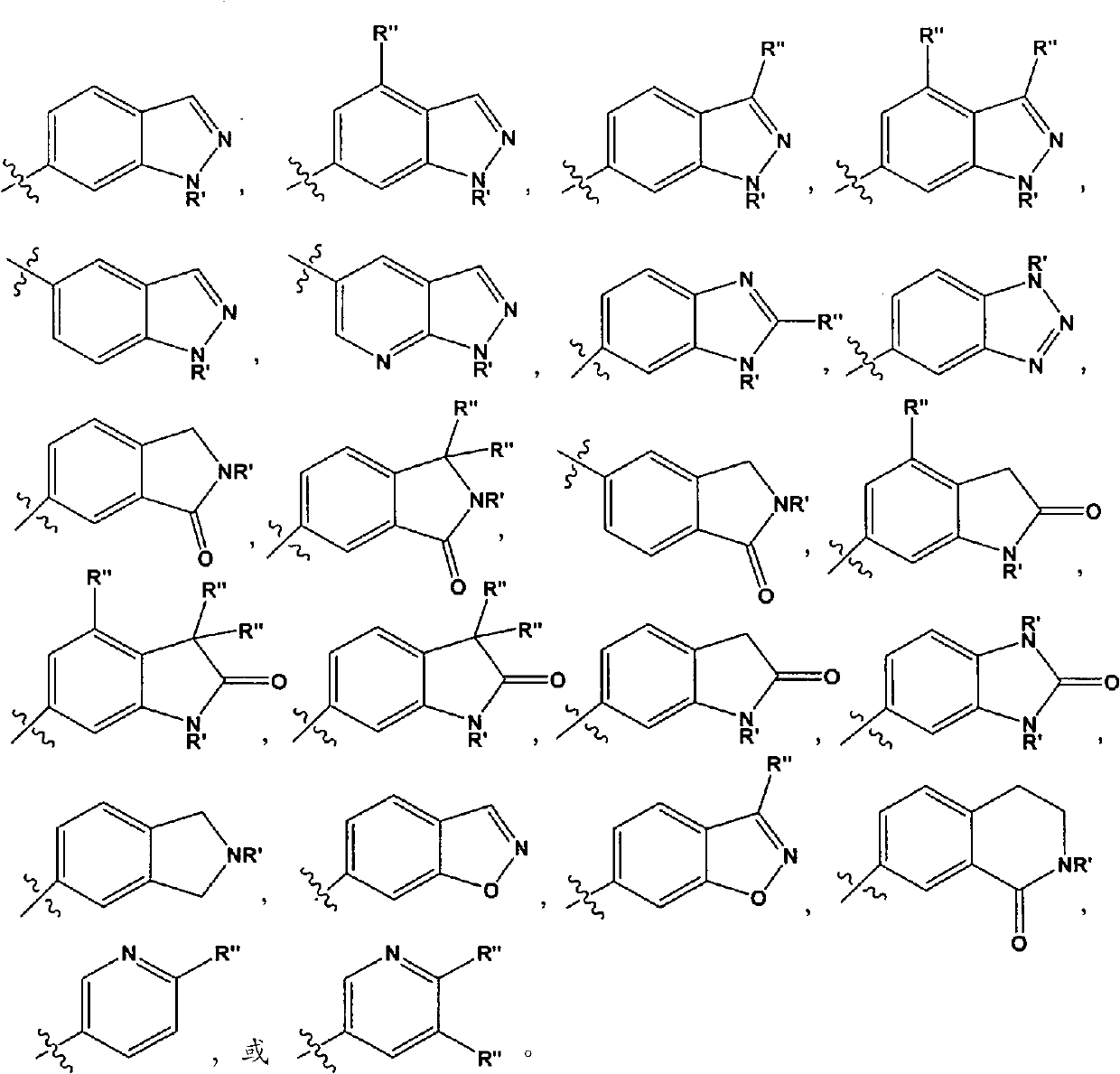
Aminotriazolopyridines and their use as kinase inhibitors
A technology of alkyl and aryl groups, applied in the field of aminotriazolopyridine and its use as a kinase inhibitor, can solve problems such as hindering the production of neuronal products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1426] Example 1: N,5-diphenyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine
[1427]
[1428] A. 2-(3-Ethoxycarbonyl-2-thioureido)-6-bromopyridine. To a solution of 2-amino-6-bromopyridine (8 g, 46.5 mmol) in dioxane (160 mL) was added dropwise ethoxycarbonyl isothiocyanate (6.09 g, 46.5 mmol) at room temperature under nitrogen. ), the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was monitored by TLC (petroleum ether:ethyl acetate=5:1). When the starting material was consumed, the dioxane was removed under reduced pressure to give crude 2-(3-ethoxycarbonyl-2-thioureido)-6-bromopyridine (14.81 g) as a solid. 1 HNMR (300MHz, DMSO-d 6 )δ (ppm) 12.16 (br s, 1H), 11.65 (br s, 1H), 8.65 (d, J=8.1Hz, 1H), 7.84 (dd, J 1 =8.1,J 2 =7.2Hz, 1H), 7.49(d, J=7.2Hz, 1H), 4.24(q, J=7.2Hz, 2H), 1.27(t, J=7.2Hz, 3H); MS(ESI): m / z 303.9[M+1] + .
[1429]B. 5-Bromo-[1,2,4]triazolo[1,5-a]pyridin-2-amine. To hydroxylamine hydrochloride (16.16g, 232.5mmol) and ...
Embodiment 2
[1432] Example 2: N-(4-morpholinylphenyl)-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine
[1433]
[1434] A. N-(4-Morpholinylphenyl)-5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine. In 5-phenyl-[1,2,4]triazolo[1,5-a]pyridin-2-amine (100 mg, 0.47 mmol), 4-(4-bromo-phenyl)-morpholine ( 137mg, 0.57mmol), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (27mg, 0.047mmol) and potassium tert-butoxide (105mg, 0.94mmol) in dioxane After degassing the mixture in (6 mL), tris(dibenzylideneacetone)dipalladium(0) (21.6 mg, 0.024 mmol) was added under nitrogen and the reaction mixture was heated at 80° C. overnight with stirring under nitrogen. The reaction mixture was quenched by adding water, and the mixture was extracted with ethyl acetate (15 mL×3). The organic layers were combined, washed with brine, dried over sodium sulfate, and concentrated. The residue was purified by preparative TLC (eluting with 25% ethyl acetate in petroleum ether) to give N-(4-morpholinophenyl)-5-phenyl-[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com