Method for producing metal hydroxide fine particle
一种氢氧化物、细粒的技术,应用在稀土金属氧化物/氢氧化物、氧化物/氢氧化物制备、氢氧化镁等方向,能够解决高成本等问题,达到小粒度、有利结晶性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067]
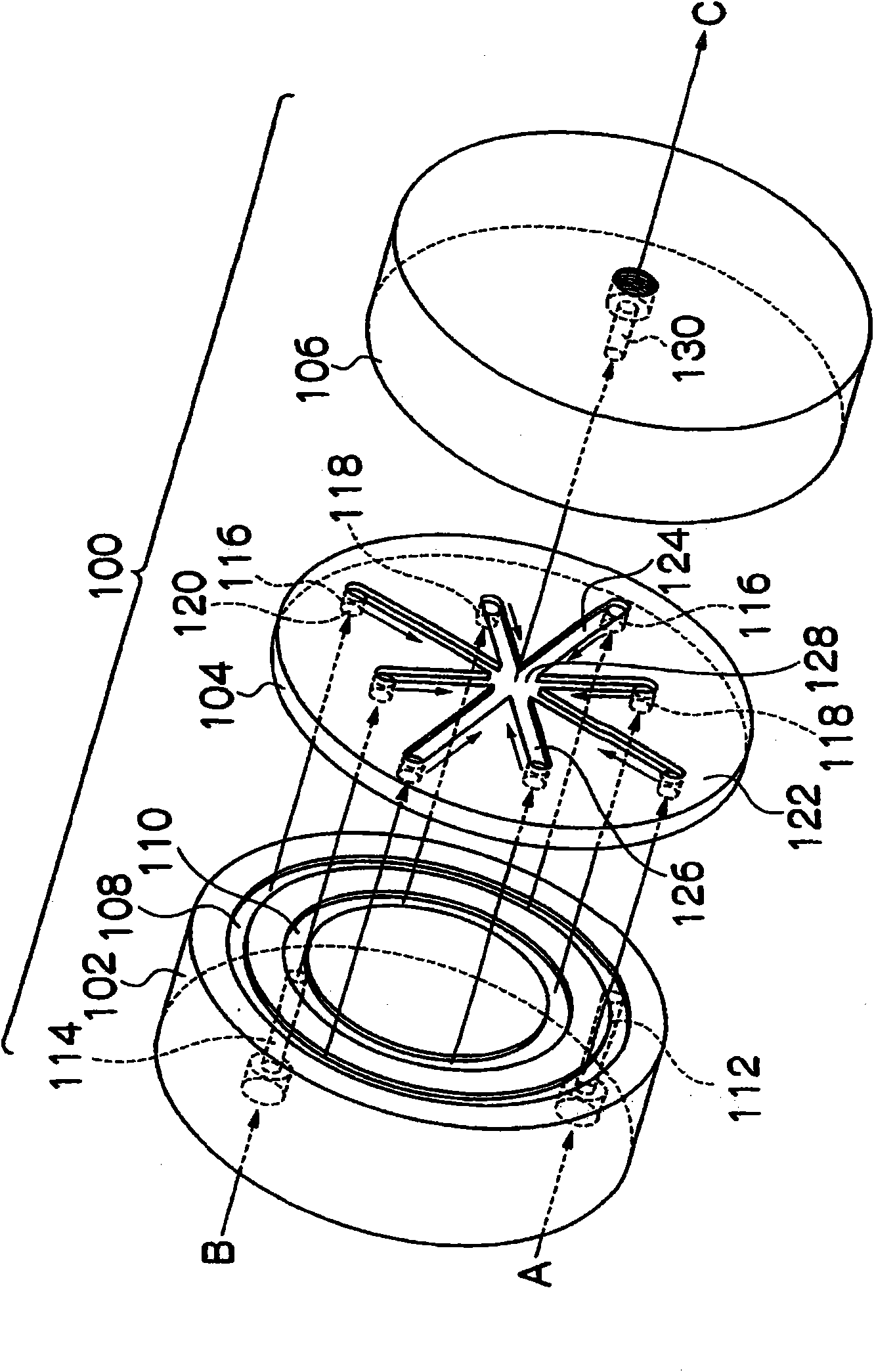
[0068] Next, referring to an embodiment of the method for producing metal hydroxide fine particles according to the present invention, the case of producing magnesium hydroxide from a sodium hydroxide solution and a magnesium chloride solution will be described as an example.
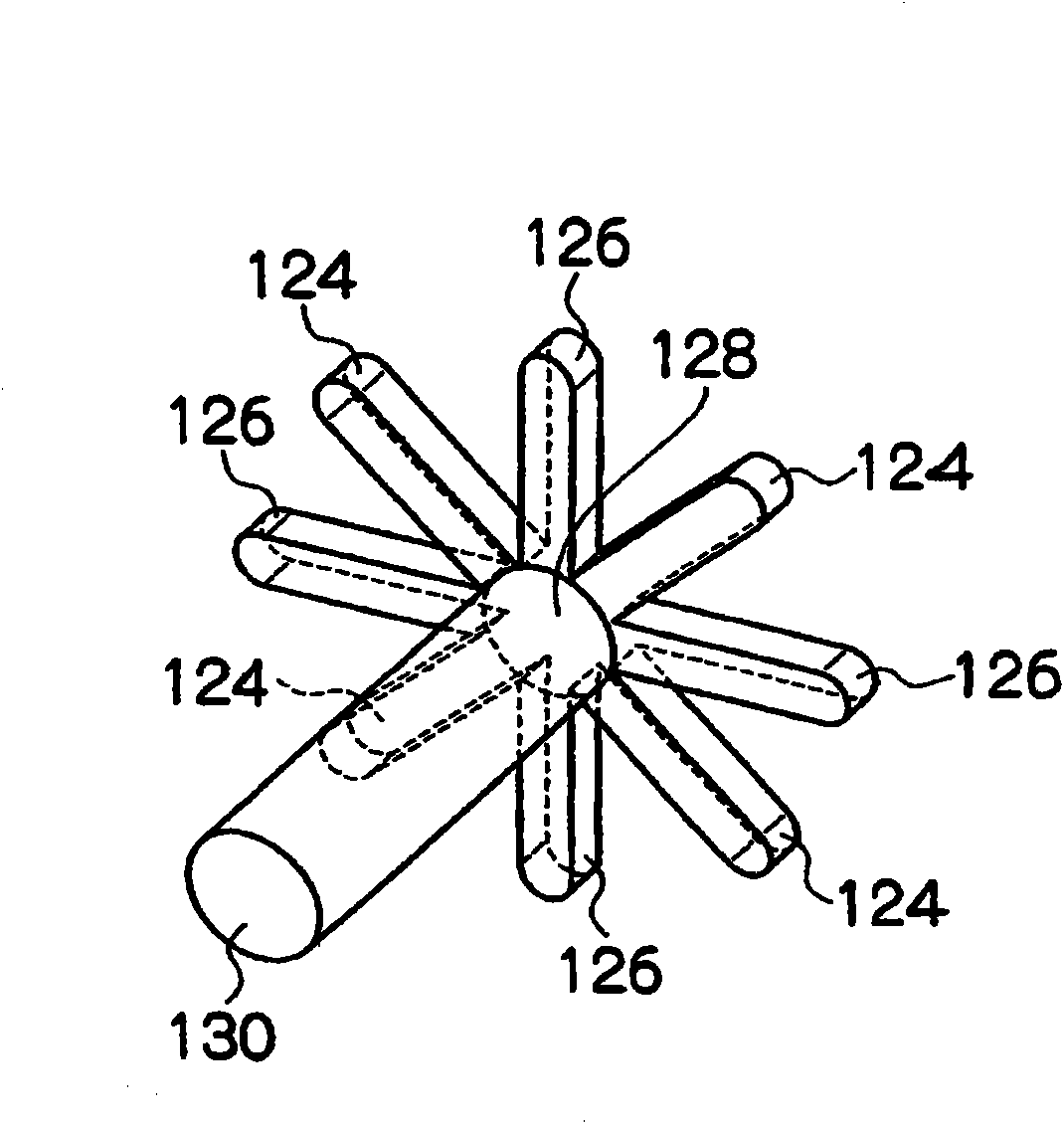
[0069] in reference figure 1 In the case of explanation, a mixed solution of a magnesium chloride solution and a silane coupling agent (3-aminopropyltrimethoxysilane) is supplied to the flow path 126 , and a sodium hydroxide solution is supplied to the flow path 124 . The solutions supplied to the flow path 126 and the flow path 124 are mixed in the mixing portion 128 and discharged to the outside of the microreactor through the perforation 130 as a discharge flow path. Thus, a reaction is induced in the mixing portion 128 and the perforations 130, thereby providing a slurry of magnesium hydroxide.
[0070] Next, the method for producing metal hydroxide fine particles according to the presen...
Embodiment 1-1
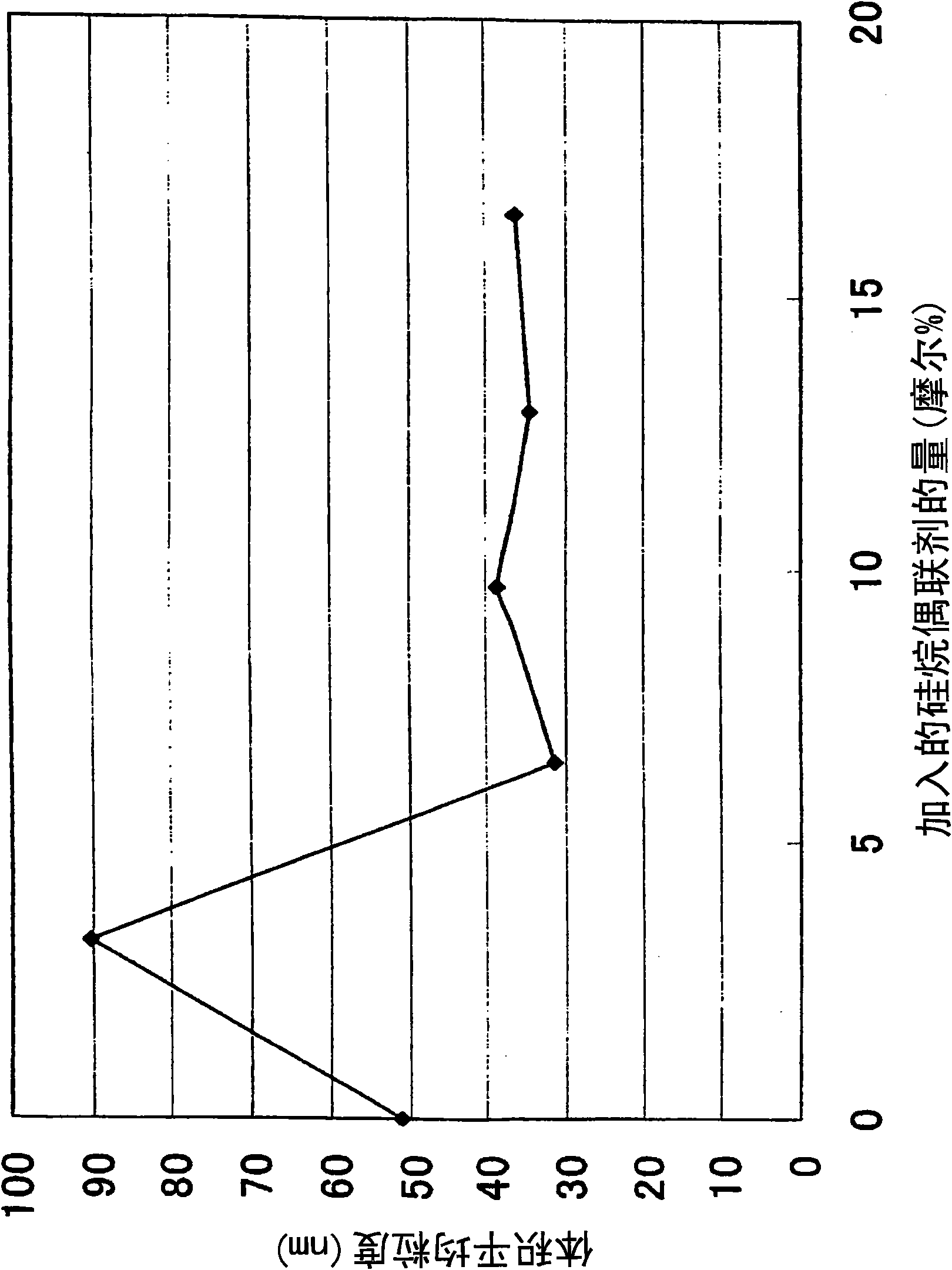
[0093] In a microreactor, at room temperature, an aqueous solution of magnesium chloride preadjusted to 0.5 mol / L and hydrogen adjusted to 3 mol / L were mixed with 3.2 mol% of 3-aminopropyltrimethoxysilane relative to magnesium ions. The sodium oxide aqueous solutions were mixed with each other at flow ratios of 200 cc / min and 100 cc / min, respectively, to obtain slurries of magnesium hydroxide. The resulting slurry was purified by washing with water until the salt concentration reached 0.00%, thereby providing a dispersion of magnesium hydroxide fine particles in water. The resulting dispersion in water to which 3-aminopropyltrimethoxysilane was added at 10% by weight relative to the magnesium hydroxide particles was heated at 120° C. for 2 hours while stirring, dried, and then redispersed to obtain hydrogen Magnesium Oxide a.
Embodiment 1-2
[0095] In a microreactor, at room temperature, an aqueous magnesium chloride solution adjusted to 0.5 mol / L and hydrogen adjusted to 3 mol / L were mixed with 6.5 mol % of 3-aminopropyltrimethoxysilane relative to magnesium ions. The sodium oxide aqueous solutions were mixed with each other at flow ratios of 200 cc / min and 100 cc / min, respectively, to obtain slurries of magnesium hydroxide. The resulting slurry was purified by washing with water until the salt concentration reached 0.00%, thereby providing a dispersion of magnesium hydroxide fine particles in water. The resulting dispersion in water to which 3-aminopropyltrimethoxysilane was added at 10% by weight relative to the magnesium hydroxide particles was heated at 120° C. for 2 hours while stirring, dried, and then redispersed to obtain hydrogen Magnesium oxide b.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com