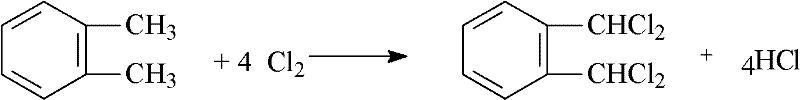Preparation process of a,a,a',a'-tetrachloroortho-xylene
A preparation process and technology of xylene, which is applied in the preparation of halogenated hydrocarbons, organic chemistry, chemical instruments and methods, etc., can solve the problems of difficult control of the chlorination end point and low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Chlorination reaction
[0018] Add 250 g of o-xylene and a benzoyl peroxide catalyst whose mass is 1-1.05% of the o-xylene mass in a 1000 mL four-neck flask. Turn on the ultraviolet lamp, start stirring, and heat with an electric heating mantle. When the temperature reaches 120°C, open the valve on the chlorine cylinder and start to pass chlorine gas. The control reaction temperature is 120~125 ℃, until the amount of feeding chlorine is 220g, sampling is analyzed by gas chromatography, at this moment, the content of trichloro-o-xylene is 60%, the content of dichloro-o-xylene is 30%, and the content of tetrachloro-o-xylene is 30%. content of 10%, and then continue to heat up to 125 ~ 130 ℃, continue to pass chlorine gas. Until the total amount of feeding chlorine is 550g, sampling is analyzed by gas chromatography. At this time, the content of trichloro-o-xylene is 30%, the content of dichloro-o-xylene is 15%, the content of tetrachloro-o-xylene is 50%, and the content ...
Embodiment 2
[0024] Chlorination reaction
[0025] Add 300 g of o-xylene and a benzoyl peroxide catalyst whose weight is 1-1.05% of the weight of the o-xylene in a 1000 mL four-necked flask. Turn on the ultraviolet lamp, start stirring, and heat with an electric heating mantle. When the temperature reaches 120°C, open the valve on the chlorine cylinder and start to pass chlorine gas. The control reaction temperature is 120~125 ℃, until the amount of feeding chlorine is 280g, sampling is analyzed by gas chromatography, at this moment, the trichloro-o-xylene content is 60%, the dichloro-o-xylene content is 30%, and the tetrachloro-o-xylene content is 30%. content of 10%, and then continue to heat up to 125 ~ 130 ℃, continue to pass chlorine gas. Until the total amount of feeding chlorine is 700g, sampling is analyzed by gas chromatography. At this time, the content of trichloro-o-xylene is 30%, the content of dichloro-o-xylene is 15%, the content of tetrachloro-o-xylene is 50%, and the cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com