Method for preparing high fullerene and special anode thereof
A fullerene and anode technology, which is applied to the preparation method of macrofullerene and the special anode field thereof, can solve problems such as difficult yield, and achieve the effects of low cost, simple production and technological process, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
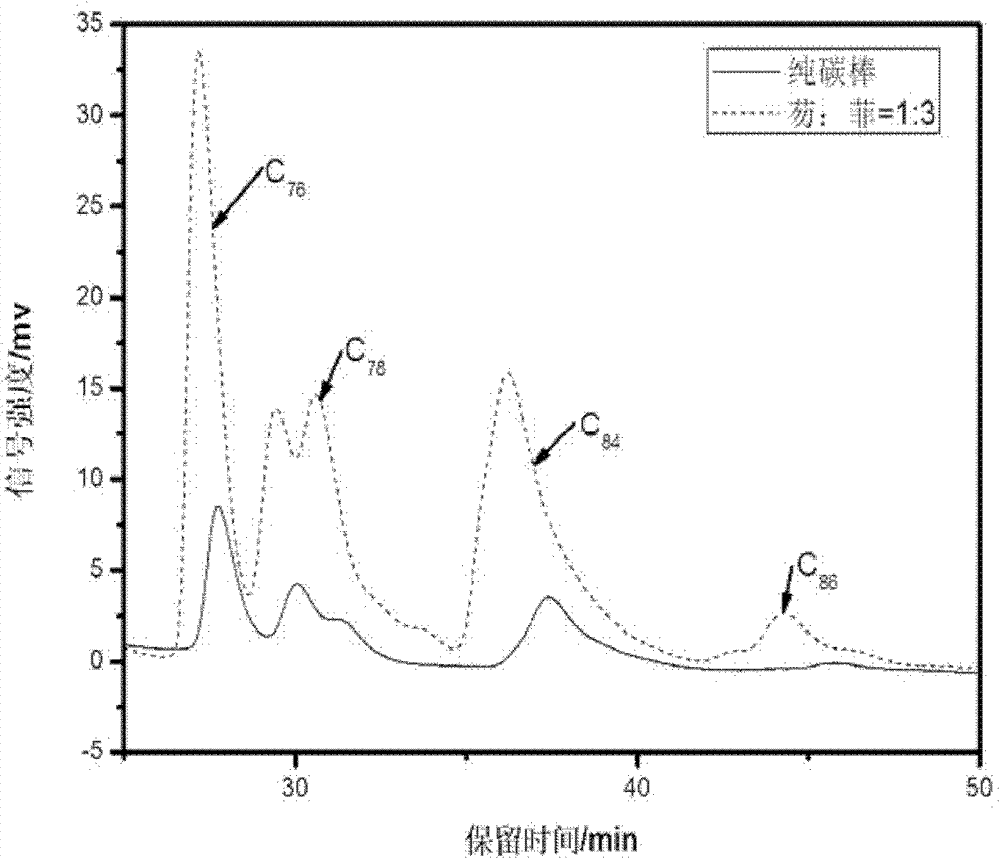
[0021] Example 1, arc discharge method to prepare large fullerenes - adding condensed aromatic hydrocarbons to the anode as fluorene: phenanthrene = 1: 3 (ratio in molar fractions)
[0022] (1) the diameter is 8mm, the length is that the solid spectrum pure graphite rod of 150mm is drilled into the hollow graphite tube that internal diameter is about 6mm, the mol fraction ratio of fluorene and phenanthrene is 1: 3 mixed ring aromatics and graphite powder The mixture is filled into the above-mentioned hollow graphite tube and compacted, wherein the mass-number ratio of the mixed condensed ring aromatic hydrocarbons of fluorene and phenanthrene to graphite powder is 1:20; the filled graphite rod is installed and fixed on the anode of the electric arc furnace, and closed Furnace cabin: turn on the vacuum pump, open the valve between the furnace cavity and the vacuum pump, and evacuate the electric arc furnace until the air pressure is lower than 10Pa, turn on the cooling circulati...
Embodiment 2
[0026] Example 2, arc discharge method to prepare large fullerenes - adding condensed aromatic hydrocarbons to the anode as fluorene: naphthalene = 3: 4 (ratio in molar fractions)
[0027] (1) The experimental procedure is the same as the step (1) in Example 1, and the difference is: the polycyclic aromatic hydrocarbon is a mixture of fluorene and naphthalene, wherein the molar ratio of fluorene and naphthalene is 3: 4; the electric current of arc discharge is 160A, the voltage is 38V, the pressure of the He atmosphere is 350Torr, and the cathode is graphite electrode;
[0028] (2) experimental procedure is with the step (2) in embodiment 1;
[0029] (3) experimental procedure is the same as step (3) in embodiment 1;
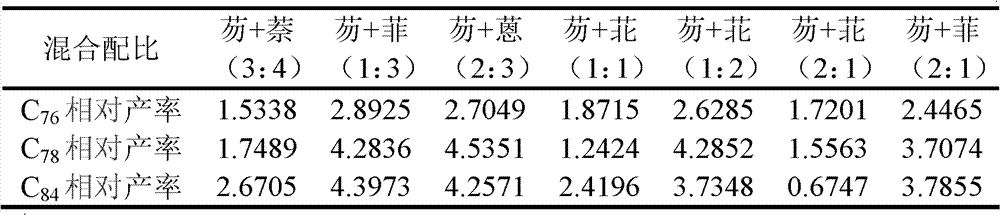
[0030] (4) Taking the peak area obtained by adding simple graphite powder in the hollow graphite rod as a benchmark (other experimental conditions are as above-mentioned steps (1)-(3)), determine the yield change of the corresponding product by the change of th...
Embodiment 3
[0031]Example 3, arc discharge method to prepare large fullerenes - adding fused ring aromatic hydrocarbons to the anode as fluorene: anthracene = 2: 3 (ratio in molar fractions)
[0032] (1) The experimental procedure is the same as the step (1) in Example 1, and the difference is: the condensed aromatic hydrocarbon is a mixture of fluorene and anthracene, wherein the molar ratio of fluorene and anthracene is 2: 3; the electric current of arc discharge is 170A, the voltage is 41V, the pressure of the He atmosphere is 450Torr, and the cathode is a tungsten electrode;
[0033] (2) experimental procedure is with the step (2) in embodiment 1;
[0034] (3) experimental procedure is the same as step (3) in embodiment 1;
[0035] (4) Taking the peak area obtained by adding simple graphite powder in the hollow graphite rod as a benchmark (other experimental conditions are as above-mentioned steps (1)-(3)), determine the yield change of the corresponding product by the change of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com