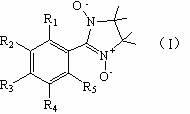
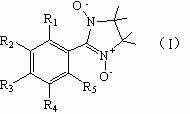
Structure of drug for treating neurodegenerative disease
A drug and composition technology, applied in the field of new drug structure, can solve problems such as obvious adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
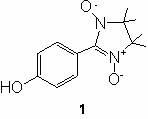
[0019] Embodiment 1: the synthetic method of compound 1
[0020]
[0021] 1.22 g (10.0 mmol) of p-hydroxybenzaldehyde and 1.48 g (10.0 mmol) of dihydroxylamine were dissolved in 50 mL of methanol and refluxed for 24 h. A large amount of white insoluble matter was formed, filtered, and the filter cake was washed with a small amount of methanol. Suspend the filter cake in 50.0 mL CH 2 Cl 2 , cooled in an ice-water bath, and added 30.0 mL NaIO 4 (1.7 g) aqueous solution, the reaction was stopped after stirring for 15 min. After static separation, the aqueous phase was washed with CH 2 Cl 2 Extracted twice, combined the organic phases, dried overnight, filtered, removed the solvent under reduced pressure, and purified by column chromatography to obtain 1.12 g of the product, with a yield of 45%. Mp: 137-139℃.R f =0.33 (CHCl 3 / CH 3 OH, 20:1). EI-MS(m / z) 250.1[M] + .IR(KBr) 3340 (OH); 1590, 1450, 1380, 880, 800, 690 cm -1 . Anal. Calcd for C 13 h 17 N 2 o 3 : C,...
Embodiment 2
[0022] Embodiment 2: the synthetic method of compound 2
[0023]
[0024] 1.88 g (10.0 mmol) of p-2,4-dichlorobenzaldehyde and 1.48 g (10.0 mmol) of dihydroxylamine were dissolved in 50 mL of methanol and refluxed for 24 h. A large amount of white insoluble matter was formed, filtered, and the filter cake was washed with a small amount of methanol. Suspend the filter cake in 50.0 mL CH 2 Cl 2 , cooled in an ice-water bath, and added 30.0 mL NaIO 4 (1.7 g) aqueous solution, the reaction was stopped after stirring for 15 min. After static separation, the aqueous phase was washed with CH 2 Cl 2 Extract twice, combine the organic phases, dry overnight, filter, remove the solvent under reduced pressure, and purify by column chromatography to obtain 1.55 g of the product with a yield of 51%. Mp: 123-125℃.R f = 0.54(CHCl 3 / CH 3 OH, 20:1). EI-MS (m / z) 302 [M] + .IR (KBr) 1590, 1450, 1365, 1000, 825, 870 cm_1. Anal. Calcd for C 13 h 15 N 2 o 2 Cl 2 : C, 51.67; H, ...
Embodiment 3
[0025] Embodiment 3: the synthetic method of compound 3
[0026]
[0027] 1.53 g (10.0 mmol) of p-fluoroformaldehyde and 1.48 g (10.0 mmol) of dihydroxylamine were dissolved in 50 mL of methanol and refluxed for 24 h. A large amount of white insoluble matter was formed, filtered, and the filter cake was washed with a small amount of methanol. Suspend the filter cake in 50.0 mL CH 2 Cl 2 , cooled in an ice-water bath, and added 30.0 mL NaIO 4 (1.7 g) aqueous solution, the reaction was stopped after stirring for 15 min. After static separation, the aqueous phase was washed with CH 2 Cl 2 Extract twice, combine the organic phases, dry overnight, filter, remove the solvent under reduced pressure, and purify by column chromatography to obtain 1.40 g of the product with a yield of 56%. Mp: 112-114℃. R f =0.52 (CHCl 3 / CH 3 OH, 20:1). EI-MS (m / z) 251 [M] + .IR (KBr) 1610, 1450, 1370, 1135, 770 cm -1 . Anal. Calcd for C 13 h 16 N 2 o 4 F: C, 55.11; H, 5.69; N, 9.88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com