Medicinal composition containing Esopiclone and preparation method thereof
A technology for eszopiclone and composition, which is applied in the field of pharmaceutical compositions containing eszopiclone, and can solve the problems of poor content uniformity, low dissolution rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
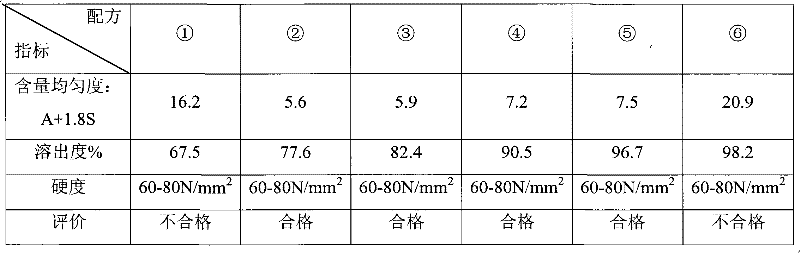
Examples
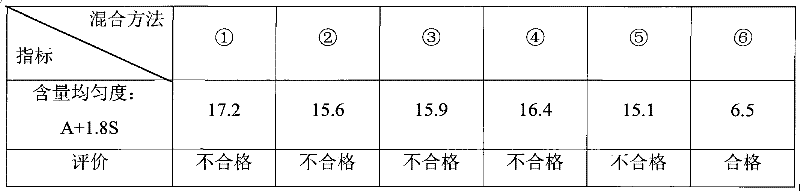
Embodiment 1 to 8
[0135] Embodiment 1 to 8, see table 17: (by weight)
[0136] Table 17 Examples 1 to 8
[0137]
[0138] The preparation method of above embodiment:
[0139] It adopts wet granulation, compresses tablets or packs capsules, and the preparation process is as follows:
[0140] (1) Take eszopiclone (with a particle size range of 50 μm-165 μm) and dextrin and mix them uniformly;
[0141] (2) Combine the sieved hydroxypropyl cellulose with disintegrating agent, starch, lactose, and microcrystalline cellulose, sieve, and mix evenly to obtain an auxiliary material mixture.
[0142] (3) Take the homogeneously mixed mixture of eszopiclone and dextrin, mix it uniformly with the mixture of auxiliary materials in an equal amount incremental method, add an appropriate amount of 10% povidone K30 solution to make a soft material, granulate, dry at 60°C, and granulate;
[0143] (4) Add micropowder silica gel and magnesium stearate, mix well, measure the content of the main drug in the gra...
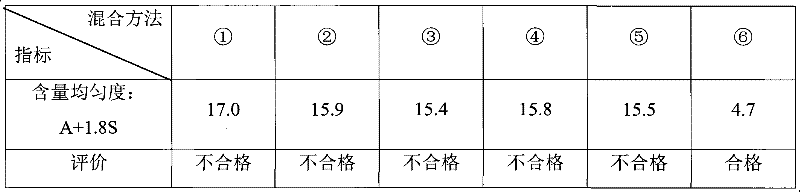
Embodiment 9 to 15
[0155] Embodiment 9 to 15, see table 20: (by weight)
[0156] Table 20 Examples 9 to 15
[0157]
[0158] The preparation method of above embodiment:
[0159] It adopts wet granulation, compresses tablets or packs capsules, and the preparation process is as follows:
[0160] (1) Take eszopiclone (with a particle size range of 50 μm-125 μm) and dextrin and mix them uniformly;
[0161] (2) Combine the sieved hydroxypropyl cellulose with disintegrating agent, starch, lactose, and microcrystalline cellulose, sieve, and mix evenly to obtain an auxiliary material mixture.
[0162] (3) Take the homogeneously mixed mixture of eszopiclone and dextrin, mix it uniformly with the mixture of auxiliary materials in an equal amount incremental method, add an appropriate amount of 10% povidone K30 solution to make a soft material, granulate, dry at 60°C, and granulate;
[0163] (4) Add micropowder silica gel and magnesium stearate, mix well, measure the content of the main drug in the g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com