Biocompatible fiber based device for guided tissue regeneration
A non-woven fiber, fiber technology used in tissue repair and regeneration to solve problems such as repairing implants, lack of strength and structural integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
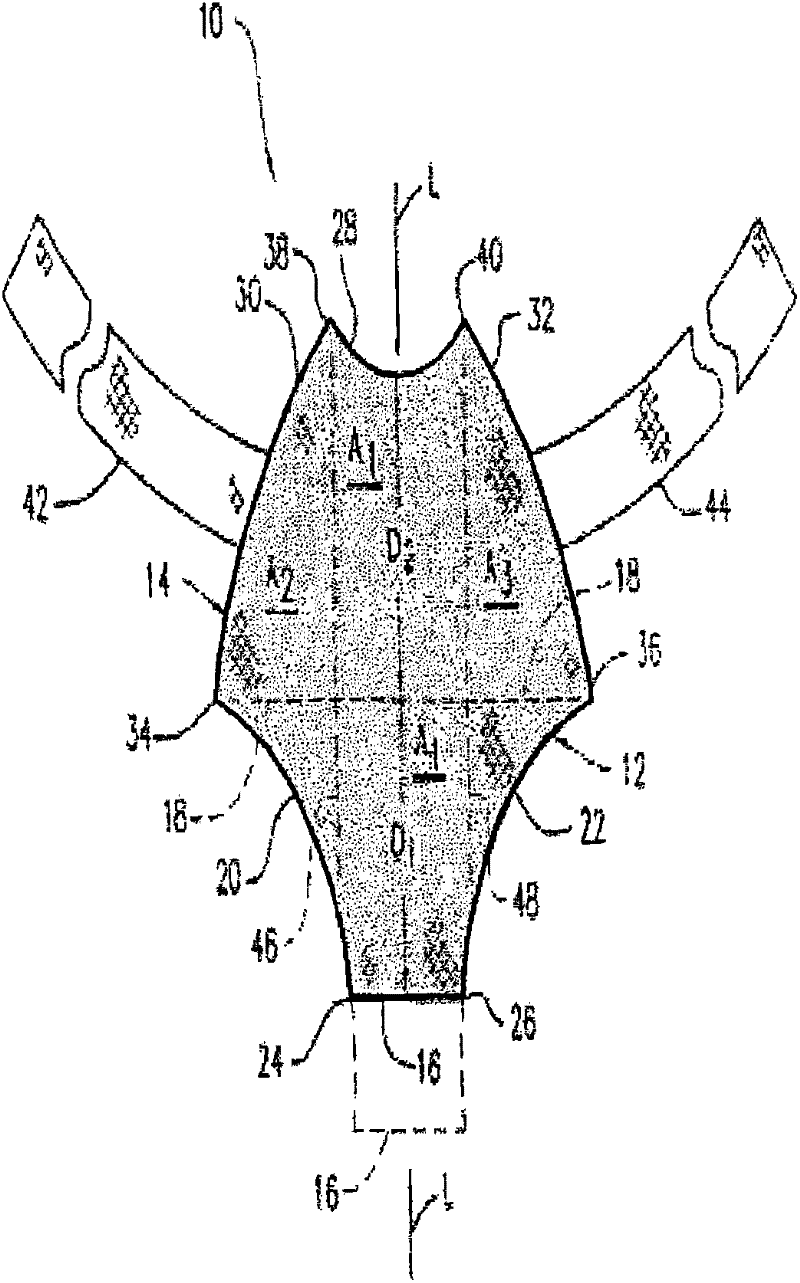
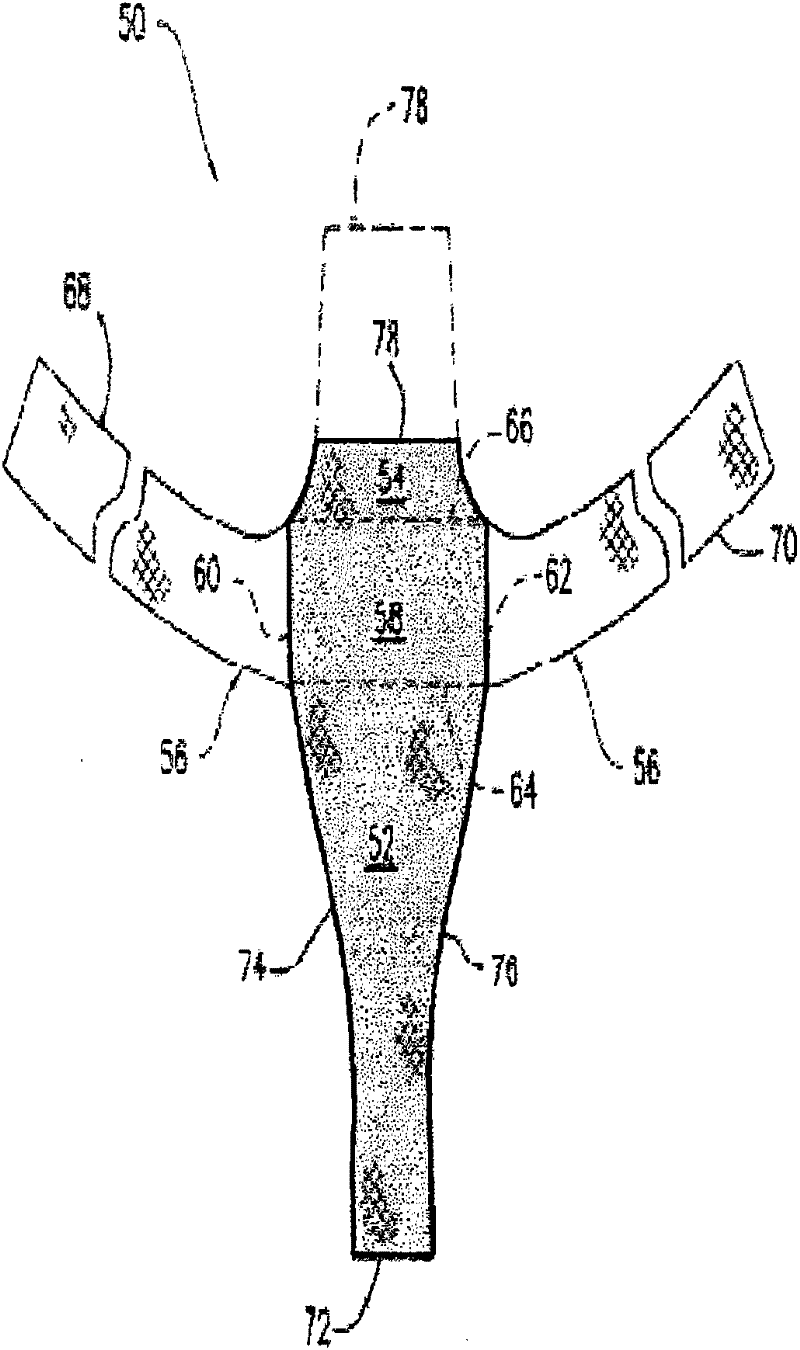
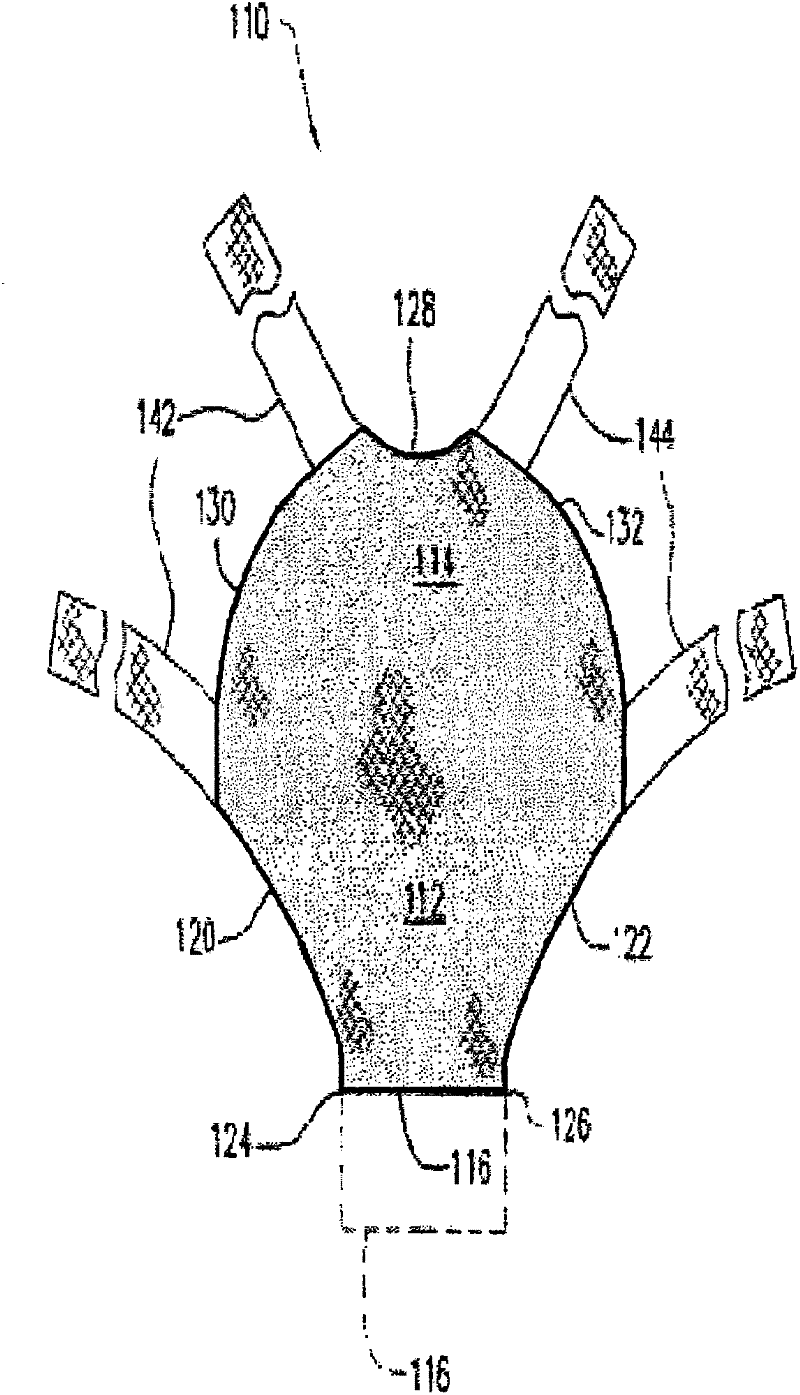
[0068] Example 1: By needle punching (GYNEMESH PS Mesh+90 / 10PGA / PLA nonwoven batting) to fabricate tissue engineering devices for pelvic floor repair
[0069] Prepared by Concordia Manufacturing, LLC (Coventry, RI) with a density of 2 mg / cm 2 90 / 10 (mol%) glycolide and lactide copolymer (90 / 10 PGA / PLA) fiber non-woven fiber mat. A 15 cm by 15 cm GYNEMESH PS mesh (Ethicon Inc, Somerville, NJ) was sandwiched between the nonwoven fibrous batts by placing a nonwoven batt on each side of the mesh. The construct is then passed through a needle loom to engage the fiber batt to embed the mesh within the nonwoven mat. Two acupuncture passes were performed to generate the device. GYNEMESH PS Mesh+90 / 10PGA / PLA nonwoven scaffold has a thickness of 1.17mm and a density of 75mg / cc.
[0070] Samples were analyzed by scanning electron microscopy (SEM). The samples were mounted on a microscope stand and a thin layer of gold was evaporated using an EMS 550 sputter coater (Electron Microsc...
example 2
[0071] Example 2: By needle punching (ULTRAPRO Mesh+90 / 10PGA / PLA nonwoven wadding Pads) to fabricate tissue engineered devices for pelvic floor repair
[0072] Prepared by Concordia Manufacturing, LLC (Coventry, RI) with a density of 2 mg / cm 2 90 / 10 PGA / PLA fiber non-woven fiber mat. A 15 cm x 15 cm ULTRAPRO mesh (Ethicon Inc, Somerville, NJ) was sandwiched between the nonwoven fibrous batts by placing a nonwoven batt on each side of the mesh. The construct is then passed through a needle loom to engage the fiber batt to embed the mesh within the nonwoven mat. Two acupuncture passes were performed to generate the device. Ultrapro mesh+90 / 10PGA / PLA non-woven scaffold has a thickness of 1.03mm and a density of 71mg / cc.
[0073] The tissue engineering device prepared according to the above steps was cut into a size of 4cm×4cm for rupture test. The burst strength of the device was evaluated using a Mullen burst test apparatus. The sample is placed in the grip area of th...
example 3
[0075] Example 3: By needle punching (90 / 10 PGA / PLA fiber: PDO fiber ratio of 50:50 GYNEMESH PS mesh + non-woven fiber batt) to manufacture tissue engineering equipment for pelvic floor repair place
[0076] The ratio of 90 / 10 PGA / PLA fiber to polydioxanone (PDO) fiber in the non-woven fiber mat is 50:50, and the density is 1mg / cm 2 , manufactured by Concordia Manufacturing, LLC (Coventry, RI). A 15 cm by 15 cm GYNEMESH PS mesh (Ethicon Inc, Somerville, NJ) was sandwiched between the nonwoven fibrous batts by placing a nonwoven batt on each side of the mesh. The construct is then passed through a needle loom to engage the fiber batt to embed the mesh within the nonwoven mat. Two acupuncture passes were performed to generate the device. GYNEMESH PS mesh + (50:50) (90 / 10PGA / PLA): PDO nonwoven scaffold with a thickness of 0.97mm and a density of 60mg / cc.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com