Slow-release particle and a production method therefor
A microparticle and controlled-release layer technology, applied in the field of controlled-release microparticles and its preparation, can solve the problems of difficult drug release control, no controlled-release microparticles, long coating time, etc., and achieve excellent dissolution properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
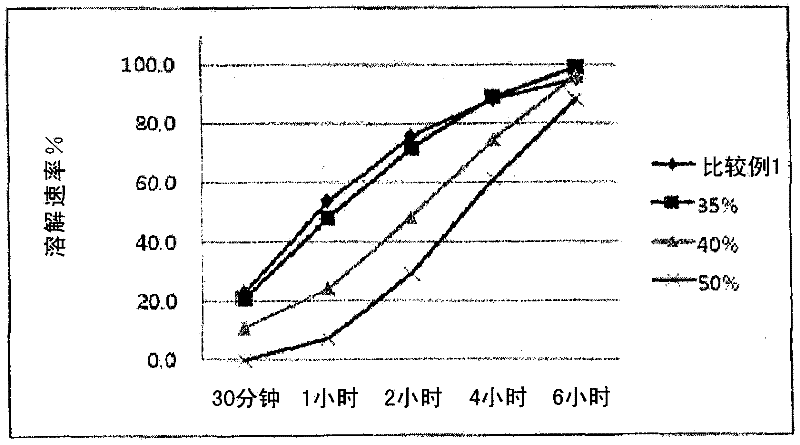
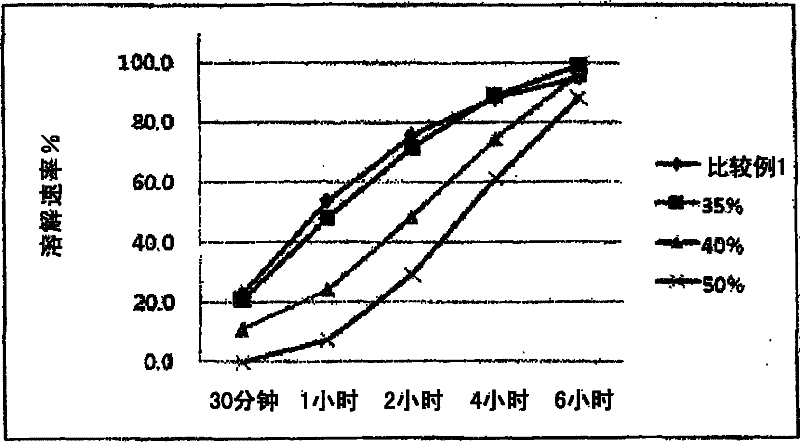
Image
Examples
Embodiment 1
[0041] Tamsulosin hydrochloride (3.33 g) was ground well and mixed with microcrystalline cellulose powder (Vivapur PH101, 496.67 g). Then, a spherical matrix containing tamsulosin hydrochloride was prepared while spraying water (500 g) with a rotary fluidized bed apparatus (GPCG-1, Glatt, Germany).
[0042] Among the particles prepared, only those with a particle diameter of 150-250 μm (60-100 mesh) were selected.
Embodiment 2
[0044] Spherical matrices were prepared in the same manner as in Example 1, except that the spray contained a dispersion of Eudragit L30D-55 (88.90 g; solids 26.67 g (solids content = 30%), water 62.23 g) and water (437.77 g) body. Only particles with a particle size of 150-250 μm (60-100 mesh) are selected.
Embodiment 3
[0046] Tamsulosin hydrochloride (3.33 g) was ground well and mixed with microcrystalline cellulose (346.67 g), calcium hydrogen phosphate (100 g) and lactose (50 g). Then, spherical substrates were prepared while spraying a dispersion comprising Eudragit L30D-55 (88.90 g; solids 26.67 g (solid content = 30%), water 62.23 g) and water (437.77 g).
[0047] Among the particles prepared, only those with a particle diameter of 150-250 μm (60-100 mesh) were selected.
[0048] Preparation of controlled release microparticles
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com