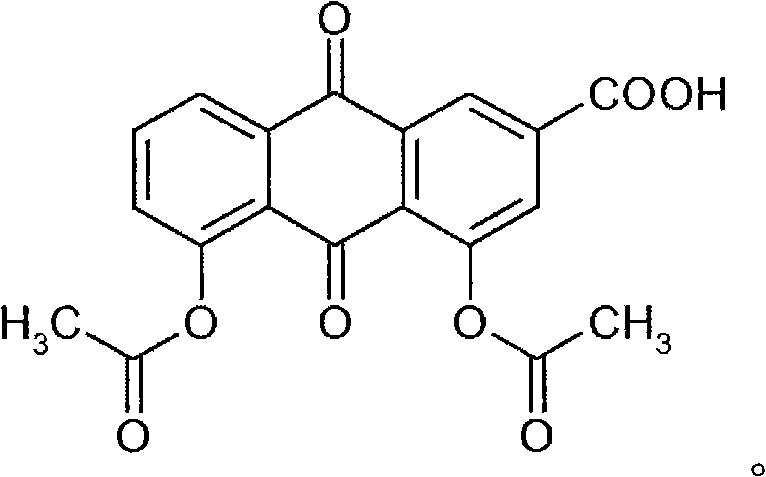
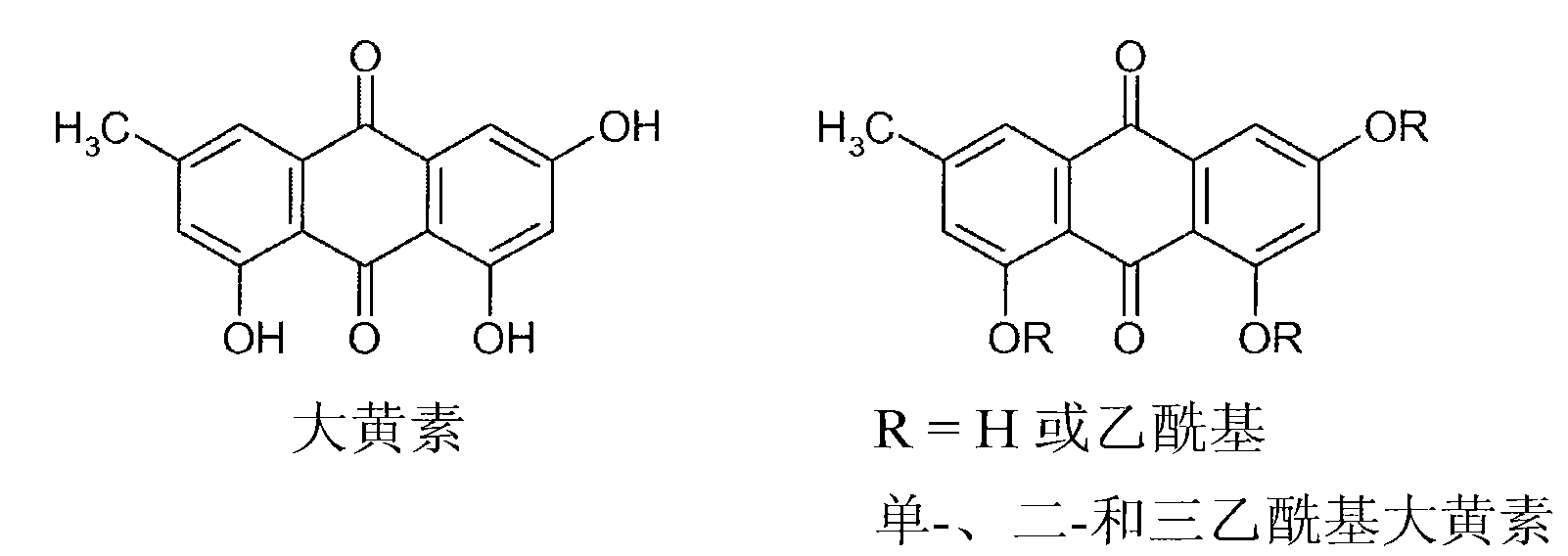
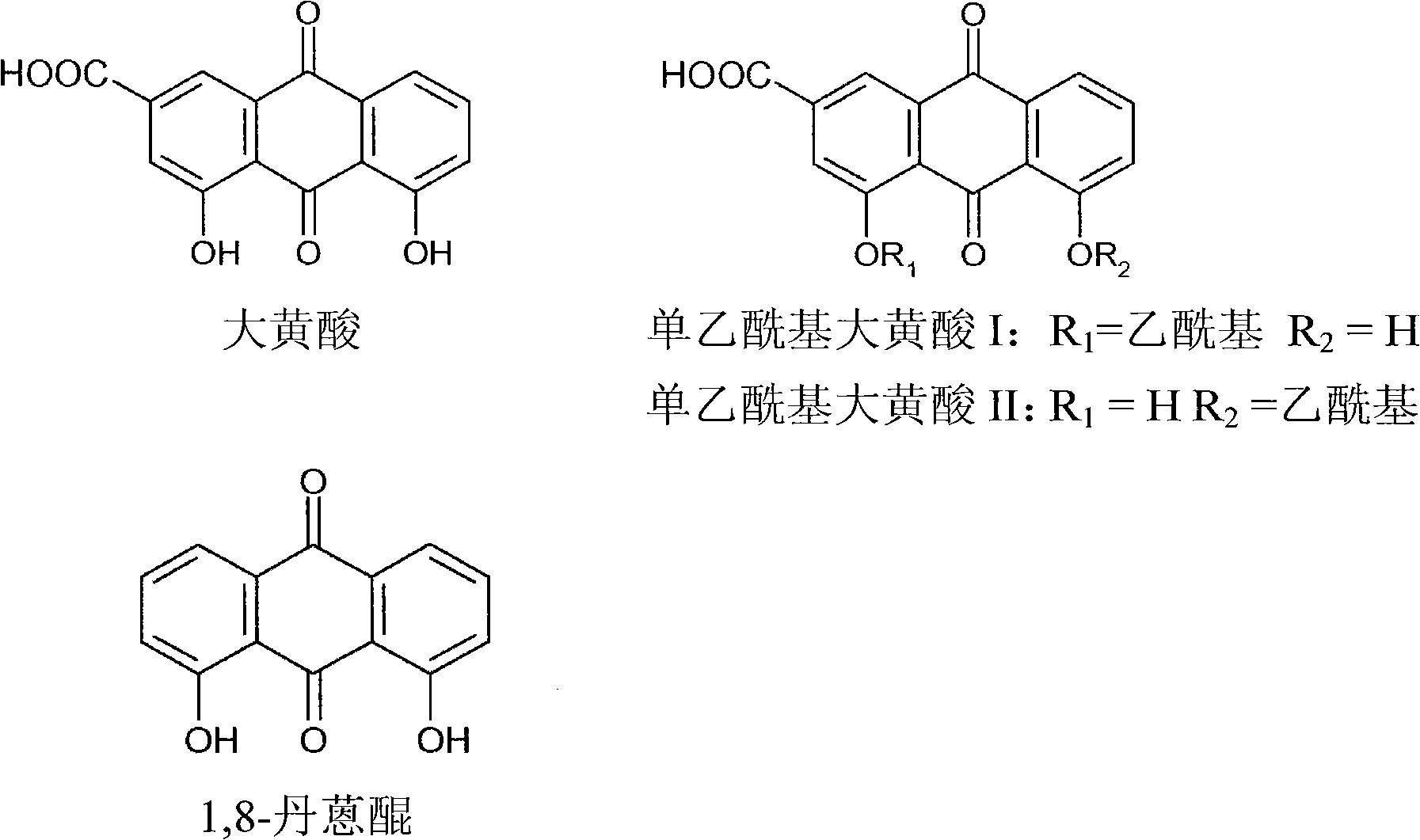
Process for the manufacture of non-genotoxic diacetylrhein (diacerein) and formulations comprising non-genotoxic diacetylrhein
A diacerein and non-gene technology, applied in the field of diacetylrhein, can solve problems such as inappropriate use of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1 (SEPABEADS SP207 and HP20 use of resin)
[0101] Preparation of Potassium Salt of Crude Diacerein
[0102] Suspend 50 g of crude diacerein containing about 500 ppm of genotoxic impurities in 750 ml of acetone and 50 ml of water, and add 25 ml of triethylamine diluted in 100 ml of acetone over a period of 3 hours under magnetic stirring, keeping pH does not exceed 7 until complete dissolution. The final solution obtained was treated with 32 g of potassium ethylhexanoate in 260 ml of acetone at 18°C. The salt-forming reagent was added over a period of 2 hours. A precipitate formed which, after filtration, was washed with 500 ml of acetone and dried under vacuum at 40° C. overnight.
[0103] Obtain 50 g of the potassium salt of diacerein.
[0104] Elution:
[0105] 15 g of this product were dissolved in 750 ml of water. After filtration under vacuum, the solution was diafiltered through 1.1 liters of SEPABEADS SP207 or DIAION HP20 A 4.5 cm-diam...
Embodiment 2
[0113] Embodiment 2 (use of DIAION HP2MG resin)
[0114] Preparation of Potassium Salt of Crude Diacerein
[0115] Suspend 50 g of diacerein containing about 500 ppm of genotoxic impurity in 750 ml of acetone and 50 ml of water and add 25 ml of triethylamine diluted in 100 ml of acetone over a period of 3 hours under magnetic stirring to maintain the pH Not more than 7 until completely dissolved. The resulting solution was treated with 32 g of potassium ethylhexanoate in 260 ml of acetone over a period of 2 hours. The resulting precipitate was filtered, washed with 500 ml of acetone and dried under vacuum at 40°C overnight.
[0116] Obtain 50 grams of diacerein potassium salt.
[0117] Elution:
[0118] Dissolve 25 grams of diacerein potassium salt in 1250 ml of water. After filtration under vacuum, the solution was diafiltered through 5.1 liters of DIAION HP2MG A 10.0 cm-diameter, 110 cm-high column (flow rate 20 ml / min).
[0119] DIAION HP2MG Typical characteristic...
Embodiment 3
[0126] 5 g of crude diacerein containing about 300 ppm of genotoxic impurity derivatives was dissolved in 40 mL of methanol, and 40 mL of water and 5 g of KOH were added under magnetic stirring. Heating to 60-65° C. is carried out in the presence of a condenser for 30 minutes; after this period, 35 ml of 6N HCL are added; dilution with about 35 ml of water is carried out and the solution is boiled for about 30 minutes. After cooling, the suspension was filtered under vacuum, the residue was washed with water and dried under vacuum to constant weight.
[0127] 4.5 g of rhein were thus obtained.
[0128] 2 g of rhein thus obtained were converted to the corresponding potassium salt as described in Example 1 for diacerein.
[0129] 2 g of the potassium salt of rhein were dissolved in 200 ml of water (final pH of the solution was 6.2). After filtration under vacuum, the solution was diafiltered through a solution containing 180 g of SEPABEADS SP207 7.5 cm-diameter, 10 cm-high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com