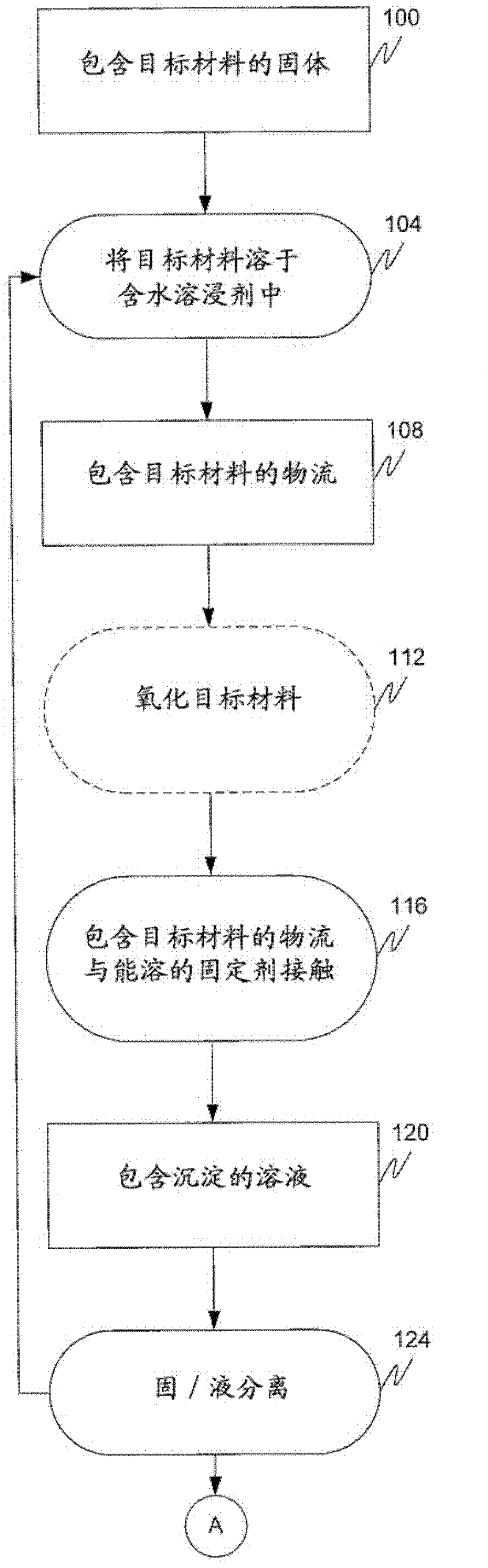
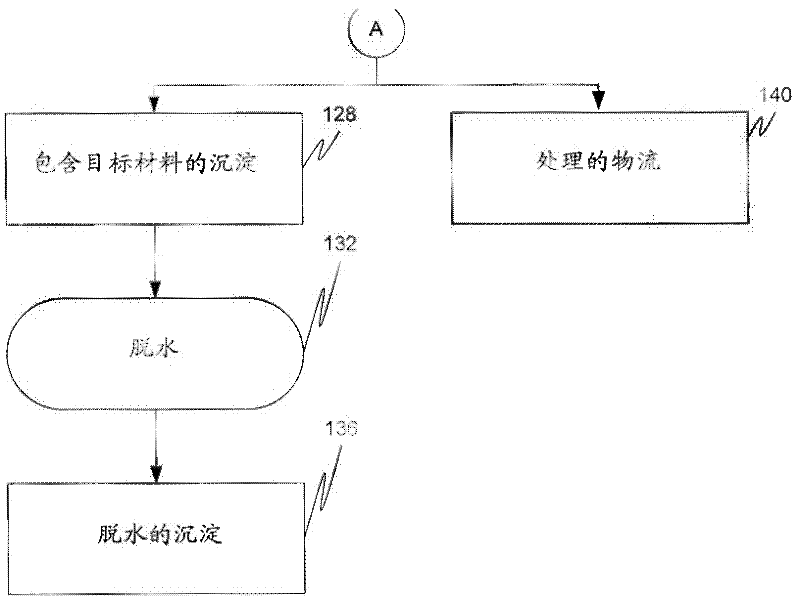
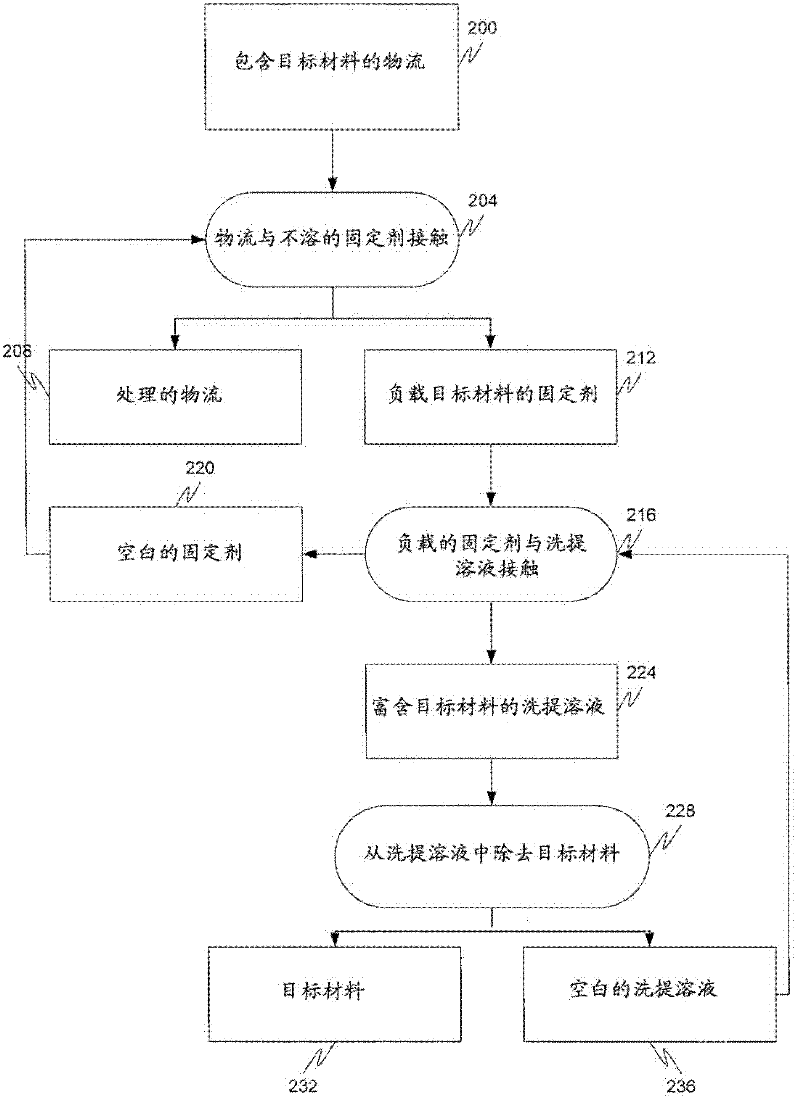
Removal of target materials using rare earth metals
A target material, rare earth technology, applied in the field of stabilization to achieve the effect of increasing flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] A set of experiments was conducted to determine the soluble cerium(III) chloride CeCl in the arsenic-containing stream 108 to reduce the arsenic concentration to less than 50 ppm 3 The maximum arsenic loading capacity. As shown in Table 1, the tested arsenic-containing stream 108 (hereinafter alkaline leach solution) had the following composition:
[0148] Table 1
[0149]
[0150] The initial pH of the seven alkaline leaching solutions was about pH 11, the temperature of the solutions was about 70-80° C., and the reaction time was about 30 minutes.
[0151] Seven alkaline leach solutions were made with different arsenic(V) concentrations as can be seen in Table 1 above. Each solution contained the same amount of sodium carbonate (20 g / L) and sodium sulfate (17.75 g / L). In the first series of experiments, 3.44 mL of cerium chloride (CeCl 3 ) is added to each isotherm and is equal to 0.918g CeO 2 (approximately 0.05 mol Ce). In the second series of experiments, ...
Embodiment 2
[0160] In another experiment, 40 grams of cerium(IV) oxide particles were loaded into a 1 inch column, resulting in a bed volume of approximately 50 ml. The ceria bed passed an arsenic-containing process stream [75% As(V), 25% As(III)] through the bed and successfully loaded the media with about 44 mg As / gram CeO 2 Or about 1,700 mg of the total amount of arsenic added to the column. Afterwards, an arsenic-supported ceria bed was passed through the bed at a flow rate of 2 mL / min for 6 bed volumes of the equivalent of a 5% NaOH solution. The solution released the 44mg As / g CeO 2 about 80% of that. Subsequently, the same cerium media was then treated again with an arsenic-contaminated process stream [75% As(V), 25% As(III)] to load the media with an additional 25 mg As / g CeO 2or another 1,000mg of arsenic. This experiment shows how to regenerate an insoluble fixative and thus extend its life, and shows that the pH of the arsenic-containing solution can be important in determ...
Embodiment 3
[0162] Trials were conducted to remove residual rare earth fixatives from alkaline leach solutions.
[0163] 15 grams of table salt (NaCl) was added to 150 mL of the alkaline leach solution containing residual cerium from the cerium nitrate addition. Table 6 shows the starting (control) and post-salt concentrations in the alkaline leach solution:
[0164] table 3
[0165] sample
[0166] As can be seen from this Table 3, 94% of the residual cerium has been removed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com