Dexamethasone sodium phosphate freeze-dried powder injection and preparation method thereof
A technology of dexamethasone sodium phosphate and freeze-dried powder injection, which is applied in the field of medicine and can solve problems such as opalescence and low clarity of dexamethasone sodium phosphate freeze-dried powder injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
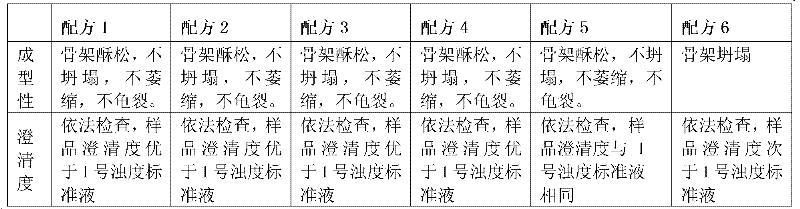
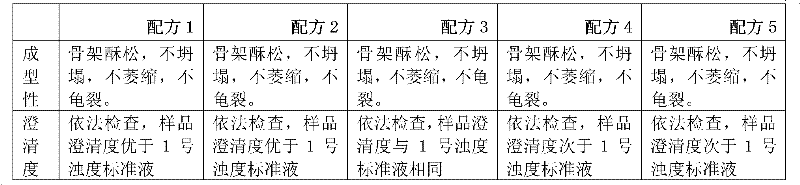
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of dexamethasone sodium phosphate freeze-dried powder injection
[0036] Dexamethasone sodium phosphate: 5g
[0037] Mannitol: 40g
[0038] Add water for injection to: 1000ml
[0039] Weigh dexamethasone sodium phosphate and mannitol according to the formula, add 800 parts by volume of water for injection, heat and stir to dissolve, add 0.2% activated carbon for needles, mix well, stir, and keep warm at 40°C to 100°C for 10 minutes to 30 minutes , coarse filtration and decarbonization, add water for injection to the filtrate to 1000 parts by volume, after passing the intermediate inspection, fine filter with a 0.22μm filter membrane, quantitatively fill in 2ml vials, press the rubber stopper halfway, and put it in a freeze-drying box Inside, freeze-dried, fully pressed rubber stopper, out of the box, locked aluminum cover, packaged, and obtained dexamethasone sodium phosphate freeze-dried powder injection.
Embodiment 2
[0040] Embodiment 2: Preparation of dexamethasone sodium phosphate freeze-dried powder injection
[0041] Dexamethasone sodium phosphate: 5g
[0042] Mannitol: 40g
[0043] Add water for injection to: 800ml
[0044] Weigh dexamethasone sodium phosphate and mannitol according to the formula, add 750 parts by volume of water for injection, heat and stir to dissolve, add 0.05% activated carbon for needles, mix well, stir, and keep warm at 40°C to 100°C for 10 minutes to 30 minutes ,, coarse filtration and decarbonization, add water for injection to the filtrate to 800 parts by volume, after passing the intermediate inspection, fine filter with a 0.22 μm filter membrane, quantitatively fill in 2ml vials, half-press the rubber stopper, and freeze-dry Inside the box, freeze-dry, fully press the rubber stopper, lock the aluminum cover when out of the box, and pack it to get dexamethasone sodium phosphate freeze-dried powder injection.
Embodiment 3
[0045] Example 3: Preparation of dexamethasone sodium phosphate freeze-dried powder injection
[0046] Dexamethasone sodium phosphate: 2g
[0047] Mannitol: 10g
[0048] Add water for injection to: 800ml
[0049] Weigh dexamethasone sodium phosphate and mannitol according to the formula, add 750 parts by volume of water for injection, heat and stir to dissolve, add 0.02% activated carbon for needles, mix well, stir, and keep warm at 40°C to 100°C for 10 minutes to 30 minutes , coarse filtration and decarbonization, add water for injection to the filtrate to 800 parts by volume, after passing the intermediate inspection, fine filter with a 0.22 μm filter membrane, quantitatively fill in 2ml vials, half-press the rubber stopper, and put it in a freeze-drying box Inside, freeze-dried, fully pressed rubber stopper, out of the box, locked aluminum cover, packaged, and obtained dexamethasone sodium phosphate freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com