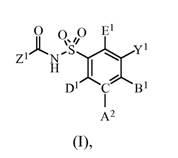
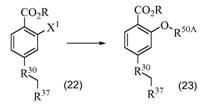
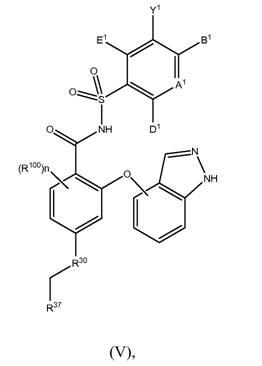
BCL-2-selective apoptosis-inducing agents for the treatment of cancer and immune diseases
A prodrug and compound technology, which is applied in the field of compounds that selectively inhibit the activity of anti-apoptotic Bcl-2 family proteins, and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[2183] 4-{4-[(4'-chloro-1,1'-biphenyl-2-yl)methyl]piperazin-1-yl}-N-({3-nitro-4-[(tetrahydro -2H-pyran-4-ylmethyl)amino)phenyl)sulfonyl)-2-phenoxybenzamide
Embodiment 1A
[2185] Tert-Butyl 4-((4'-chlorobiphenyl-2-yl)methyl)piperazine-1-carboxylate
[2186] Combine 4'-chlorobiphenyl-2-carbaldehyde (Example 27C) (4.1g), tert-butyl piperazine-1-carboxylate (4.23g), and sodium triacetoxyborohydride (5.61g) / CH 2 Cl 2 (60ml) (and) Stir for 24 hours. The reaction was quenched with methanol and poured into ether. The solution was washed with water and brine, concentrated, and chromatographed on silica gel with 2-25% ethyl acetate / hexane.
Embodiment 1B
[2188] 1-((4'-Chlorobiphenyl-2-yl)methyl)piperazine
[2189] In CH 2 Cl 2 (30ml) and trifluoroacetic acid (30mL), stir Example 1A (3.0g) and triethylsilane (1ml) 2 Hours, and the reaction was concentrated, and then absorbed in ether and concentrated again. The product is used without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com