Application of gossypol derivative to preparing anti-tumor medicament
A technology for drugs and tumors, applied in the field of biomedicine, can solve the problems of non-drugs and poor water solubility of Apogossypol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
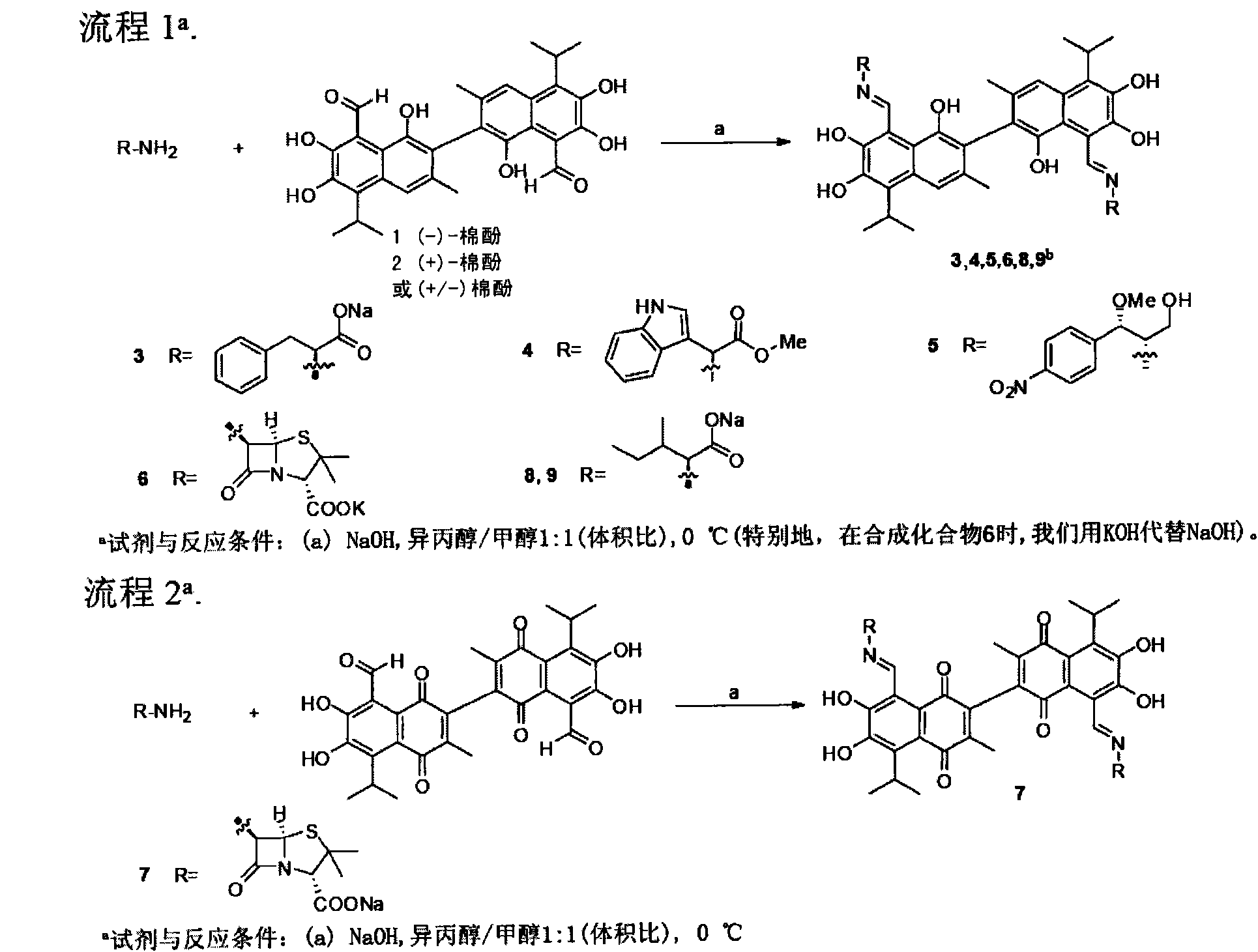
[0086] 1. Compound Preparation
[0087] A general procedure for the preparation of the compounds can be found in figure 1 , the method description is as follows:
[0088] Preparation of 6-APA-Na-vassinone (Compound 7)
[0089]Take 39 mg of 6-APA (6-aminopenicillin alkanoic acid), 10 mg of sodium hydroxide, dissolve in 6 ml of methanol and isopropanol [1:1 (v / v)] at 0 degrees Celsius, and detect when the pH is close to 6 , 48 mg of cottonone was added, kept at room temperature for 5 hours, filtered with suction, rinsed with isopropanol, and dried to obtain 64 mg of compound 7.
[0090] Compound 7
[0091] 1 H NMR (300MHz, CD 3 OD) δ9.54(s, 2H), 9.53(s, 2H), 5.65(d, J=6Hz, 2H), 5.66(d, J=5.7Hz, 2H), 5.44-5.46(m, 4H) , 4.28 (br s, OH), 4.25 (br s, OH), 3.99 (m, 4H), 1.96 (s, 6H), 1.98 (s, 6H), 1.66 (s, 6H), 1.66 (s, 6H) , 1.59(s, 12H), 1.44(d, J=6.9Hz, 12H), 1.43(d, J=7.2Hz, 12H); 13 C NMR (75MHz, CDCl 3 )δ187.3, 187.3, 185.4, 185.3, 172.8, 171.2, 171.1, 167.4, 167.2, ...
Embodiment 1
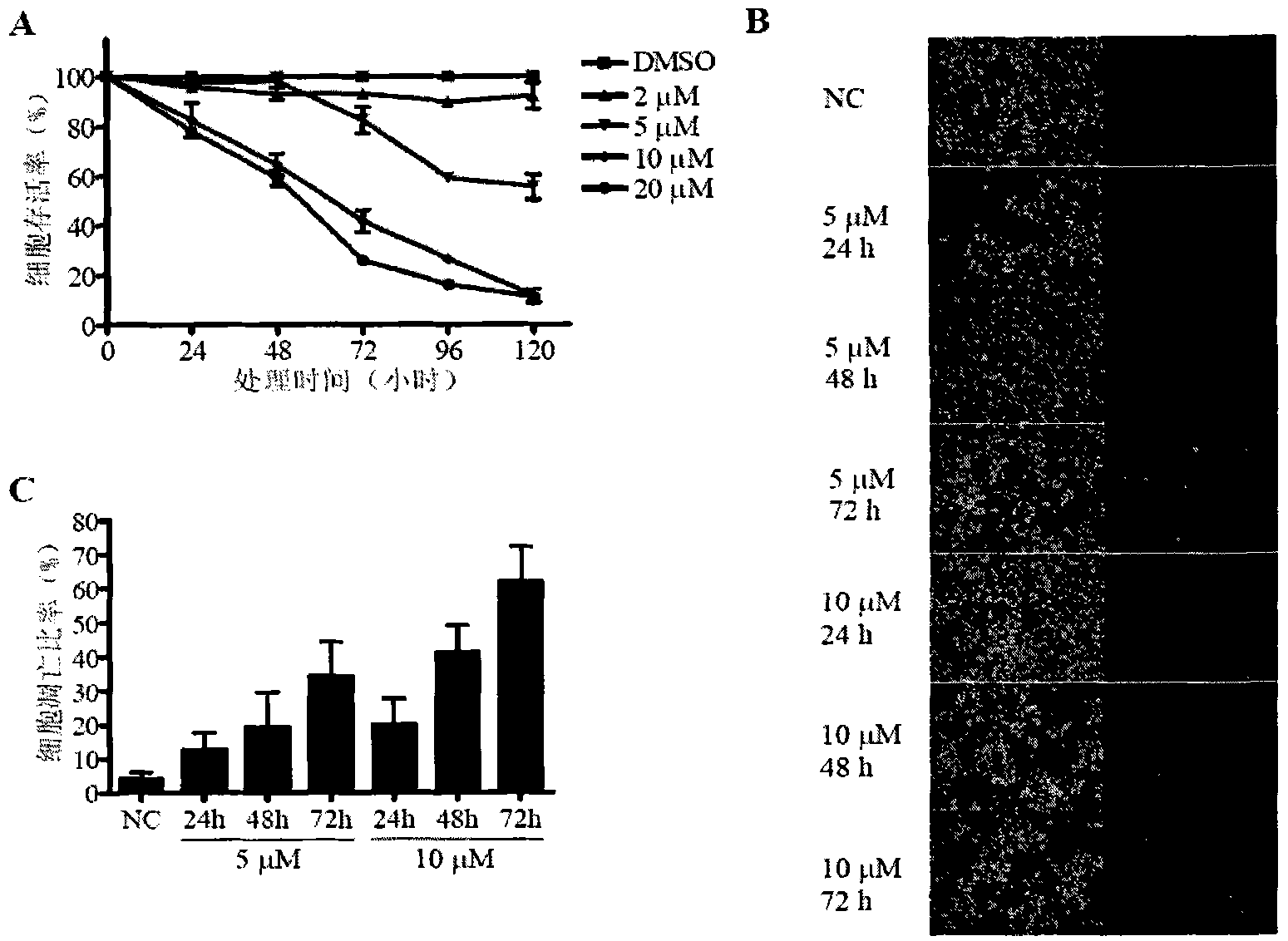
[0135] Example 1. Cell Viability Detection
[0136] The inventors first detected the newly synthesized compound ( figure 1 , compound 3-9; wherein compound 8 is an optical racemate, compound 9 is a positive optical compound) killing effect on different tumor cell lines. After some human and mouse tumor cell lines were treated with different concentrations of compounds for 72 hours, cell viability was detected by MTT assay.
[0137] The results showed that compounds 3-9 all had a certain killing effect on tumor cells, especially compound 7. Human and mouse colon cancer cell lines SW620, HCT116 and CT26 are sensitive to compound 7, the median lethal dose IC 50 Approximately 6 to 18 μM (Table 1). Breast cancer cell lines MDA-MB-435, MCF-7 and 4T1 are also sensitive to compound 7, IC 50 Values over 28 μM. Compound 7 also had obvious killing effects on mouse melanoma cell line B16-F10, human lung cancer cell line A549 and prostate cancer cell line PC-3 (Table 1). These resu...
Embodiment 2
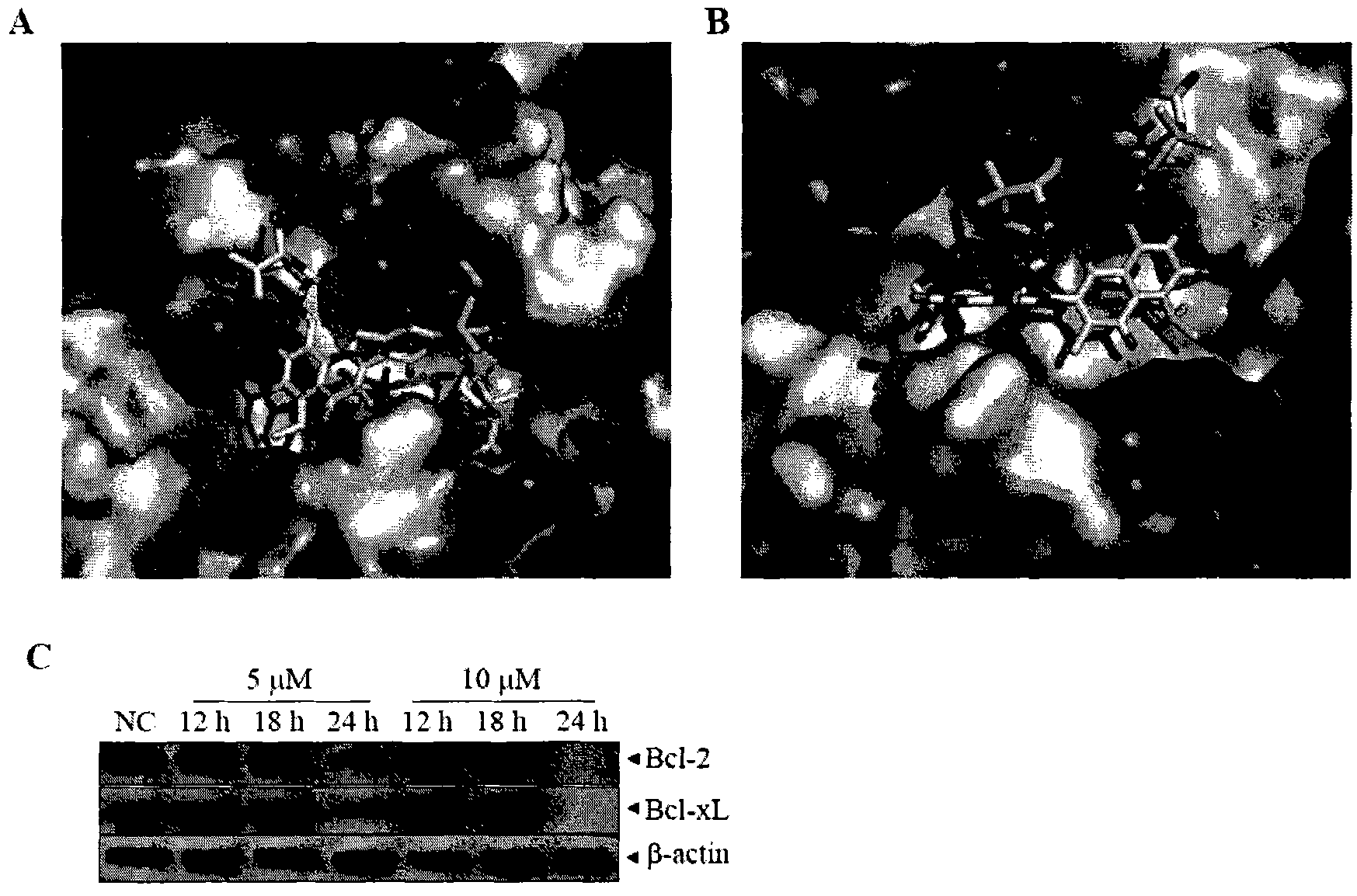
[0141] Example 2. Compound 7 induces apoptosis of CT26 cells
[0142] The present inventors treated CT26 cells with different concentrations of compound 7 for 24, 48 or 72 hours, and detected the apoptosis rate of tumor cells by TUNEL kit ( figure 2 B). See TUNEL statistical results figure 2 c.
[0143] The above results show that compound 7 can induce tumor cell apoptosis, and the induction of tumor cell apoptosis has time- and dose-dependent effects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com