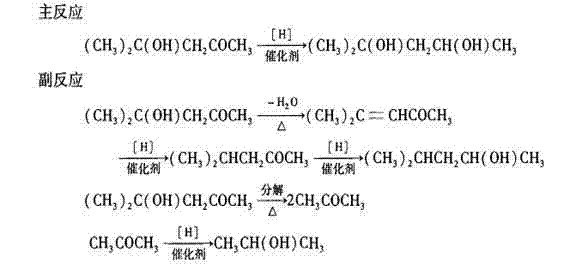
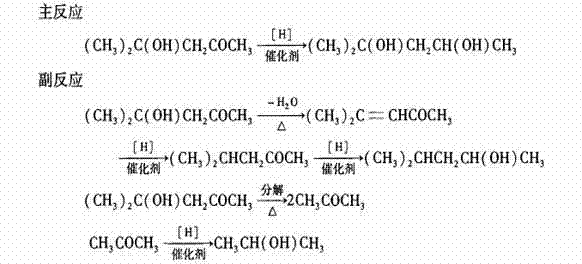
Process for synthesizing 2-methyl-2,4-pentendiol through hydrogenation reduction of diacetone alcohol
A technology of isohexanediol and diacetone alcohol, which is applied in the production process of isohexanediol, can solve the problems of difficult operation, product yield of only 86%, high production cost, etc., and achieve the effect of reducing unit consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] A specific example of implementation is given below, but the technical solution protected by the present invention is not limited to this example.
[0026] Using 2000 L of diacetone alcohol as raw material hydrogenation reduction synthesis of isohexanediol, its specific process steps are:
[0027] Step 1: Start the vacuum pump to evacuate the raw material metering tank. When the vacuum is greater than -0.06Mpa, close the pumping valve, open the feeding valve, and mix 2000 L of diacetone alcohol raw material and 200ppm of sodium bicarbonate and pump it in. Raw material metering tank.
[0028] Step 2: Open the vacuum valve on the reduction pot, pump the reduction pot to a vacuum of -0.1Mpa, then open the feed valve on the reduction pot, and add 2000 L diacetone alcohol raw material and 200ppm sodium bicarbonate from the metering tank To reduction pot, start stirrer to stir simultaneously, add the Raney nickel catalyst of 95Kg.
[0029] Step 3: Close the vacuum valve, op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com