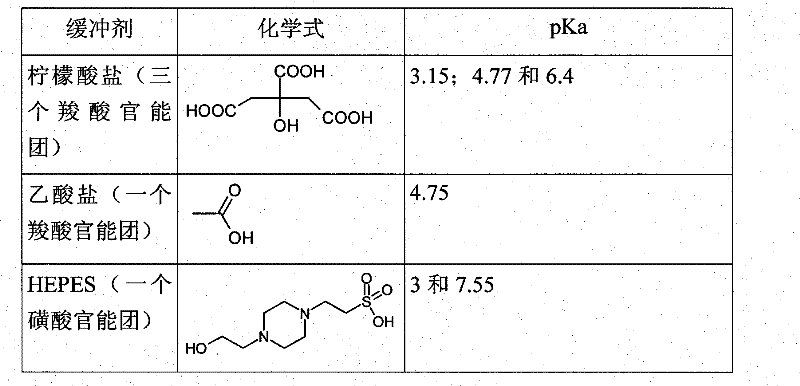
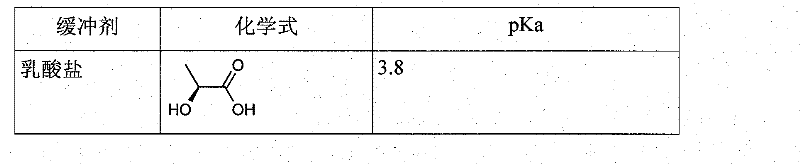
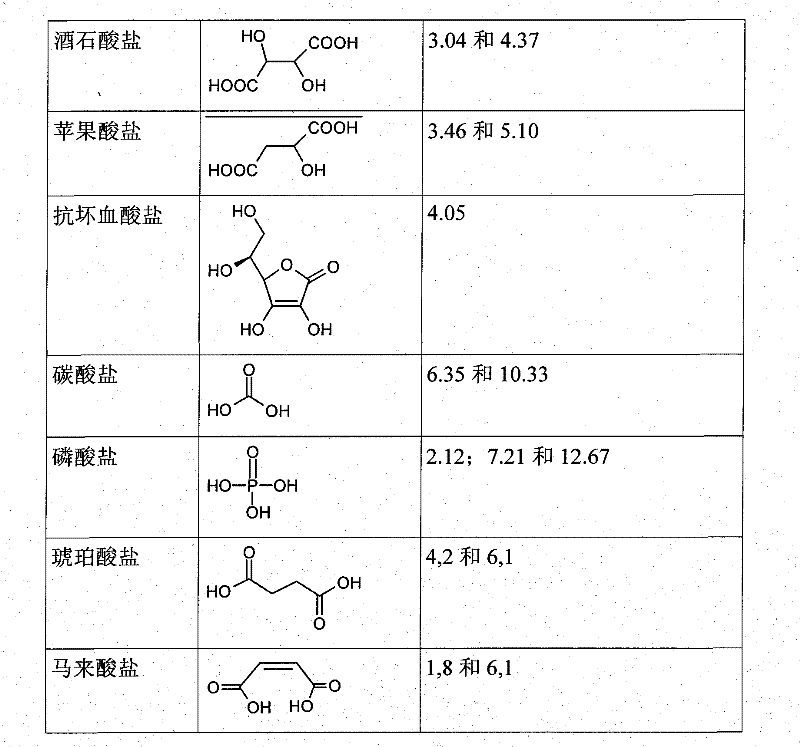
Use of buffers for radionuclide complexation
A technology of radionuclide and buffer, applied in the field of contrast agent composition, can solve problems such as damage and degradation of carrier chelate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0216] - illustrates the use of NOTA and PCTA chelates as well as folate and peptide biocarriers for the preparation of supported chelates (chelates + biocarriers) (emphasized that one skilled in the art can thus use many other biocarriers, many other linkers, and many other chelates to prepare similar compounds).
[0217] Example 1: Comparing complexation of buffers
[0218] These buffers were prepared at pH 4. They are obtained by mixing 0.1M weak acid with 0.1M weak base.
[0219] To illustrate (similar protocol used other chelates) the steps for NOTA and PCTA chelates are detailed:
[0220] 50mg of NOTA or PCTA (0.16mmol) was dissolved in 5ml of 0.1M buffer. Add 0.5ml of 0.5M 69 GaCl 3 (1.5eq) solution. The reaction medium is stirred at room temperature. Samples were taken periodically for 15 minutes and analyzed by LC / MS.
[0221] In the table below, "fully complexed" means that the chelate has successfully complexed gallium.
[0222] Positive results were obtai...
Embodiment 2
[0229] Embodiment 2: the synthesis of didenitrogen-NOTA:
[0230]
[0231] step 1:
[0232]K at 0.6g 2 CO 3 Dissolve 0.5 g of Intermediate 2 in 20 ml of CH in the presence of 3 CN. Will be in 20ml CH 3 A suspension of the brominated derivative (int.1) in CN was added. The reaction medium is maintained at reflux for 18 hours under argon and under vigorous magnetic stirring. After returning to ambient temperature, the reaction medium is filtered. The insoluble material was taken up in 20 ml of water, followed by filtration. The filtrate was evaporated under pressure. The resulting residue was absorbed in Et 2 O, and then filtered. 1.2 g of product are obtained. [M+H]+=423.16.
[0233] Step 2:
[0234] 0.6 g of the intermediate obtained in the previous step was suspended in 2.4 ml of ethanol. Dissolution was complete after adding 6 ml of 1M NaOH. The reaction medium is stirred at 70° C. for 1 hour and 30 minutes (1H30). After returning to ambient temperature,...
Embodiment 3
[0250] Example 3: Synthesis of folate-NOTA:
[0251] a) a compound of the formula:
[0252]
[0253] 20g (91.7mmol) of Boc 2 O dissolved in 40 ml of CH 2 Cl 2 middle. Add 22g (366.6mmol) dropwise in 200ml of CH 2 Cl 2 diaminoethane solution in . The reaction vials were mixed for 2 hours at room temperature. First, the product is purified by extraction with water. in Na 2 SO 4 The organic layer was dried and filtered. Then, with CH 2 Cl 2 It was purified by flash chromatography on silica gel with a gradient of methanol / methanol. 4 g of a yellow oil are obtained. m / z = 161 (ES+).
[0254] b) a compound of the formula:
[0255]
[0256] Dissolve 10.27g (24mmol) of Fmoc-Glu-OtBu in 300ml of CH 2 Cl 2 middle. 2.8 g of NHS and 4.98 g of DCC were introduced. After 45 minutes, the reaction mixture was filtered and CH dissolved in 50 ml was added dropwise 2 Cl 2 A solution of 3.869 g of the product obtained in a) in . After 2 hours at room temperature, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com