Novel composite active cardinal dye and manufacturing method thereof
A reactive, deep red technology, applied in the field of dyes, can solve the problems of not being able to be used for printing, unable to dye the color, and unable to pull out the white background, and achieve the effects of low directivity, bright color and good low temperature solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
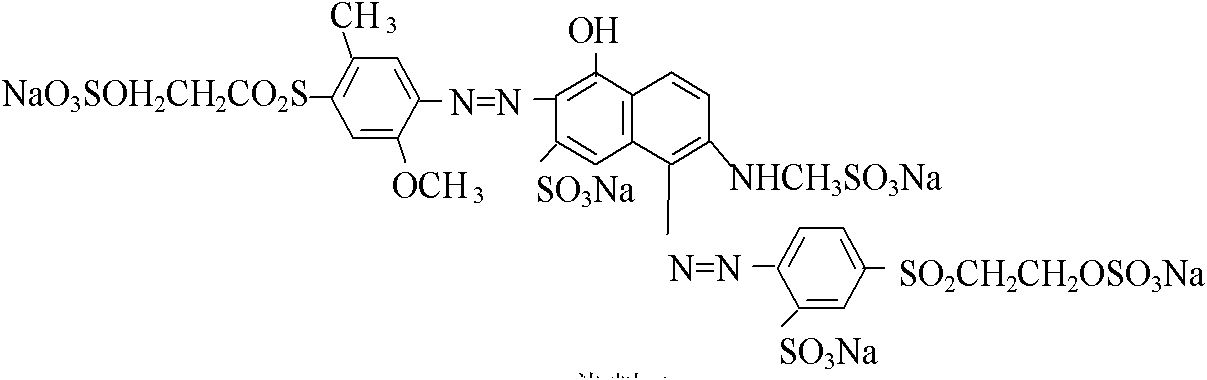
[0025] Example 1: 1 mole of sulfonated para-ester was reacted with hydrochloric acid and sodium nitrite at a temperature of 0 to 10°C to obtain a diazonium salt, and the diazonium salt was mixed with 0.5 mole of J acid and 0.5 mole of sulfonated methyl The mixed solution of J acid sodium salt is subjected to a coupling at 5-15° C. under acidic conditions to obtain a coupled material.
[0026] 1 mole of 2-methoxy-5-methyl-p-beta-sulfate ethyl sulfone aniline is reacted with hydrochloric acid and sodium nitrite at a temperature of 0-5°C to obtain a diazonium salt. The diazonium salt and the above-mentioned coupling material are subjected to secondary coupling at a temperature of 8-15° C. and a pH of 5.5-7.5. After concentration by nanofiltration and spray drying, the structural components of formula I and formula II (deep red original powder) are obtained.
[0027] Diazotize 2-amino-6-(2-sulfate ethylsulfone)-1-naphthalenesulfonic acid with hydrochloric acid and sodium nitrite a...
Embodiment 2
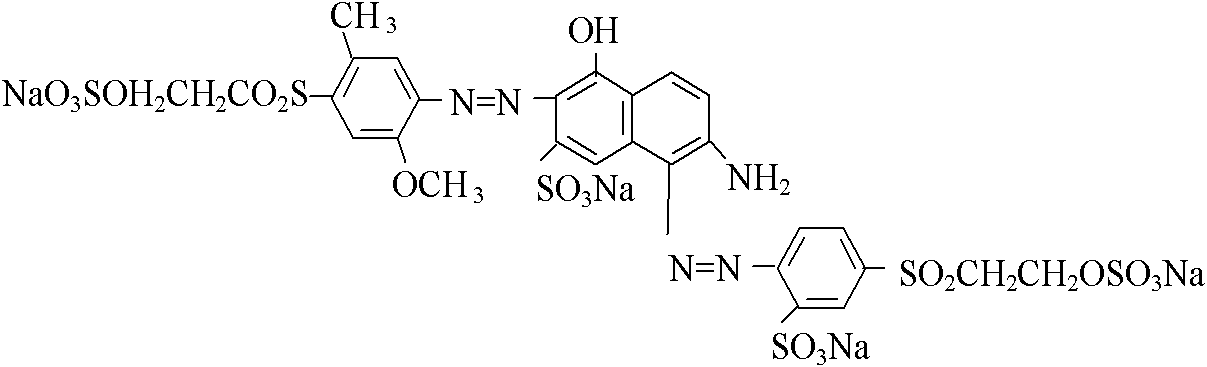
[0029] Example 2: 1 mole of sulfonated para-ester was reacted with hydrochloric acid and sodium nitrite at a temperature of 0 to 10°C to obtain diazonium salt, and diazonium salt was mixed with 0.55 mole of J acid and 0.45 mole of sulfonated methyl J Mixed solution of acid sodium salt at 5-15°C, conduct a coupling under acidic conditions, and then reconstitute 1.01 moles of 2-methoxy-5-methyl-p-β-sulfate ethyl sulfone aniline with hydrochloric acid and sodium nitrite Nitrogen reaction, temperature 0 ~ 5 ℃. The prepared diazonium salt is subjected to secondary coupling with the above-mentioned coupling material at a temperature of 8 to 15° C. and a pH of 5.5 to 7.5, concentrated by nanofiltration and sprayed to dryness to obtain the structural components of formula I and formula II (original crimson powder).
[0030] Diazotize 2-amino-6-(2-sulfate ethylsulfone)-1-naphthalenesulfonic acid with hydrochloric acid and sodium nitrite to prepare diazonium salt and add N-benzoyl H aci...
Embodiment 3
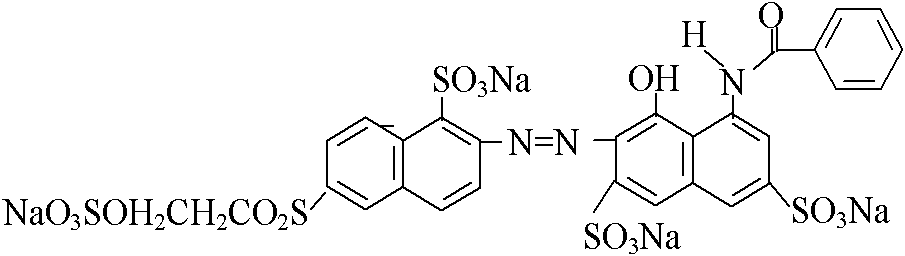
[0032] Example 3 1 mole of sulfonated para-ester was subjected to diazonium reaction with hydrochloric acid and sodium nitrite at a temperature of 0-10°C to obtain a diazonium salt. Diazonium salt and 1.0 mole sulfonated methyl J acid solution 5 ~ 15 ℃, carry out a coupling under acidic conditions, and then use 1.01 mole 2-methoxy-5-methyl-p-β-sulfate ethyl sulfone aniline Hydrochloric acid and sodium nitrite undergo diazonium reaction at a temperature of 0-5°C. The prepared diazonium salt is subjected to secondary coupling with the above-mentioned coupling material at a temperature of 8-15° C. and a pH of 5.5-7.5. After concentration and spray drying, the structural component of formula I (deep red powder 1) is obtained.
[0033] 1 mole of sulfonated para-ester is reacted with hydrochloric acid and sodium nitrite at a temperature of 0 to 10°C to obtain a diazonium salt. 15°C, carry out a coupling under acidic conditions to obtain the coupled material, and then add 1.01 moles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| color fastness | aaaaa | aaaaa |
| soaping fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com