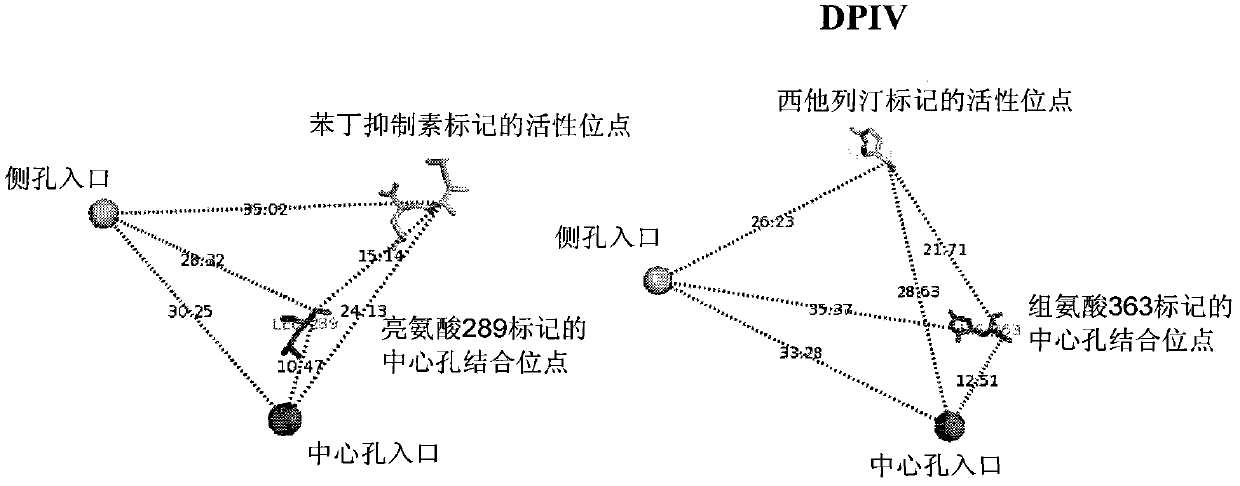
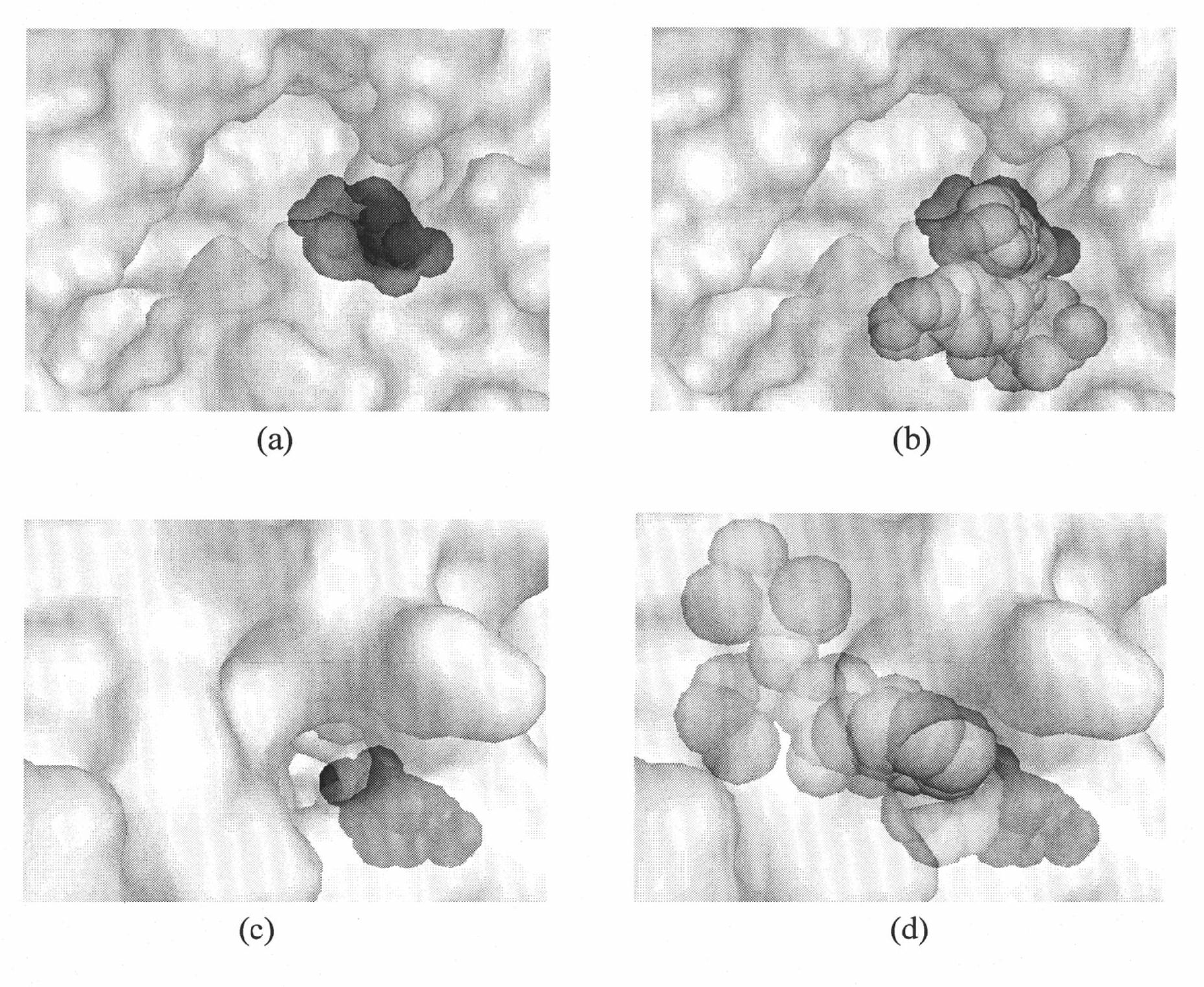
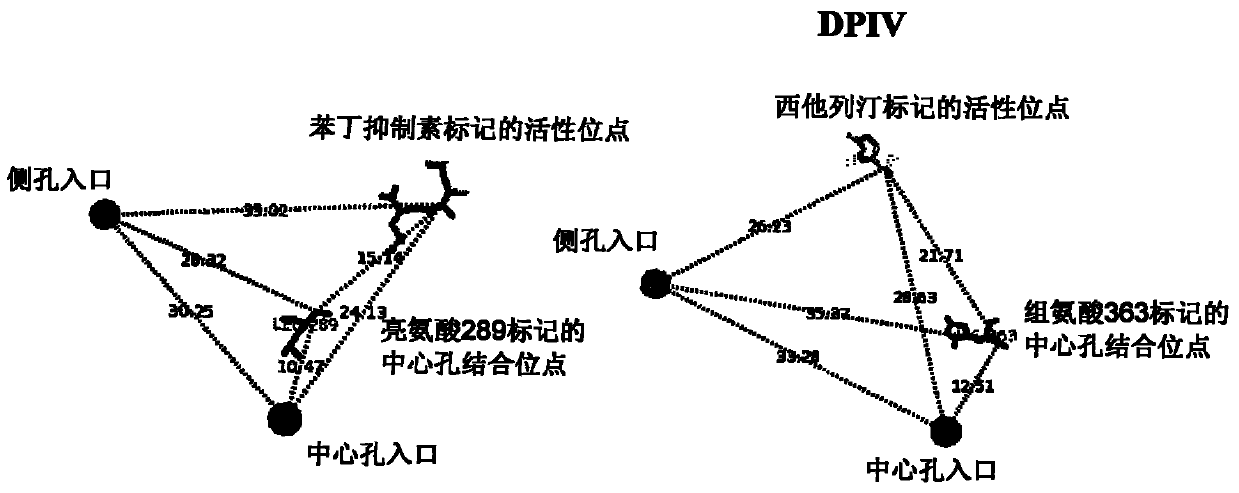
Dual alanyl-aminopeptidase and dipeptidyl-peptidase iv inhibitors
A compound, fused technology, applied in the field of preparation of novel dual inhibitors of DPIV and APN, which can solve problems such as weakening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0225] Preparation of compounds of general formula (I)
[0226] For the preparation of compounds of general formula (I), the following synthetic routes of Schemes 1 to 17 were selected; the reaction conditions for selected steps are shown in the following schemes:
[0227] plan 1:
[0228]
[0229] Reagents and conditions: a) 1. LiAlH 4 , THF, rfl. (reflux); 2.NaOH / H 2 O, Boc 2 O, DCM, RT; b) 1, (COCl) 2 , DMSO, DCM, -78°C to >-10°C; 2. Vinyl-magnesium bromide, DCM, THF, RT; c) Ac 2 O, pyridine, DCM, 0°C to >RT; d) NaIO 4 , cat. (catalytic amount) RuCl 3 , CH3CN, EtOAc, H 2 O; e) DCC, HOBt, DCM, 17,0°C to > RT; f) LiOH, MeOH, H 2 O; g) aq. (aq.) HCl (37%), EtOH.
[0230] Scenario 2:
[0231]
[0232] Reagents and conditions: a) H 2 , Pd / C, MeOH; b) NaH, NaI, PMB-Cl, THF; c) LiOH, MeOH, H 2 O; d) n-C 7 h 13 COCl, TEA, DMAP, DCM, RT; e) f)
[0233] Option 3:
[0234]
[0235] Reagents and conditions: a) RCOCl, TEA, DMAP, DCM; b) LiOH, MeOH, H 2 O; c) RO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com