Application of farrerol, derivative thereof and pharmaceutically-acceptable salts of farrerol and derivative to medicine for treating heart cerebrovascular disease caused by vasoconstriction
A cardiovascular and cerebrovascular disease, vasoconstriction technology, applied in cardiovascular system diseases, drug combinations, medical preparations containing active ingredients, etc. , the effect of low price
Inactive Publication Date: 2012-03-28
SHANXI MEDICAL UNIV
View PDF1 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] There are no relevant reports at home and abroad on the pharmacological effects of DJS-1 on the prevention and treatment of cardiovascular and cerebrovascular diseases caused by vasoconstriction.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
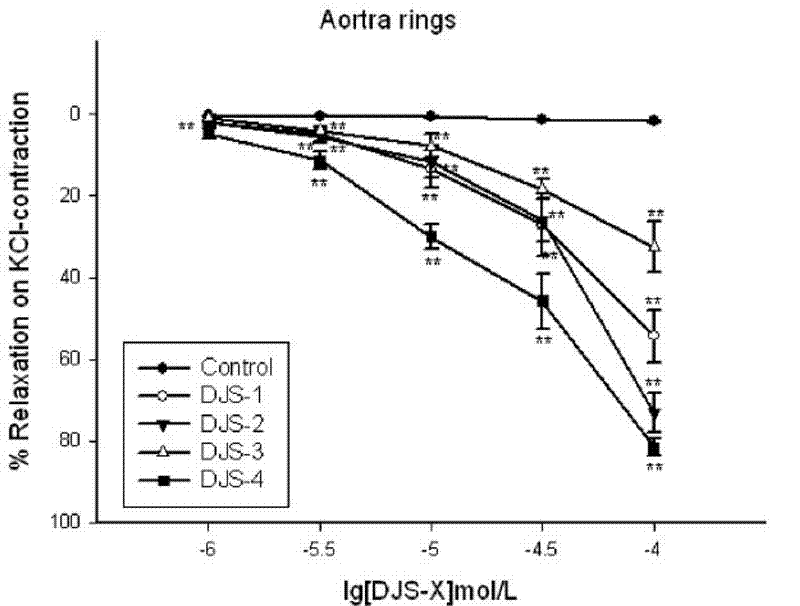
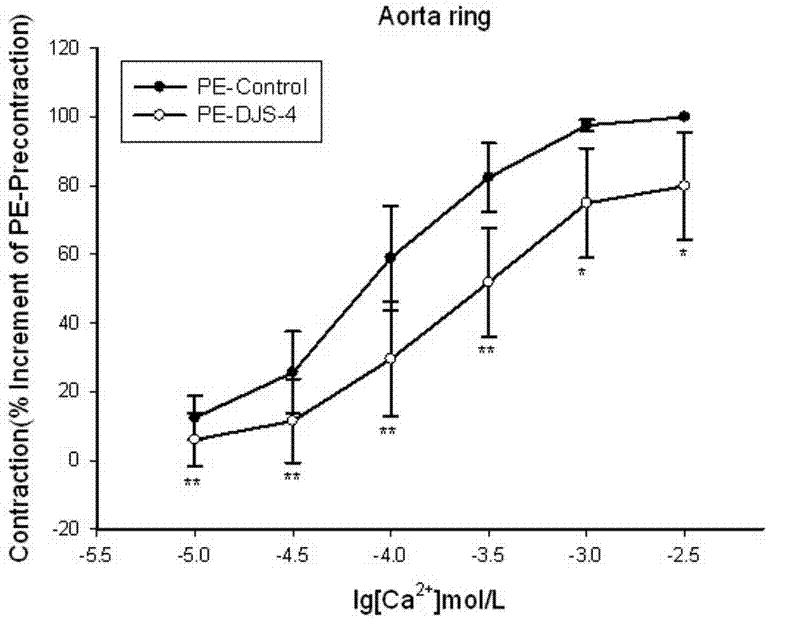
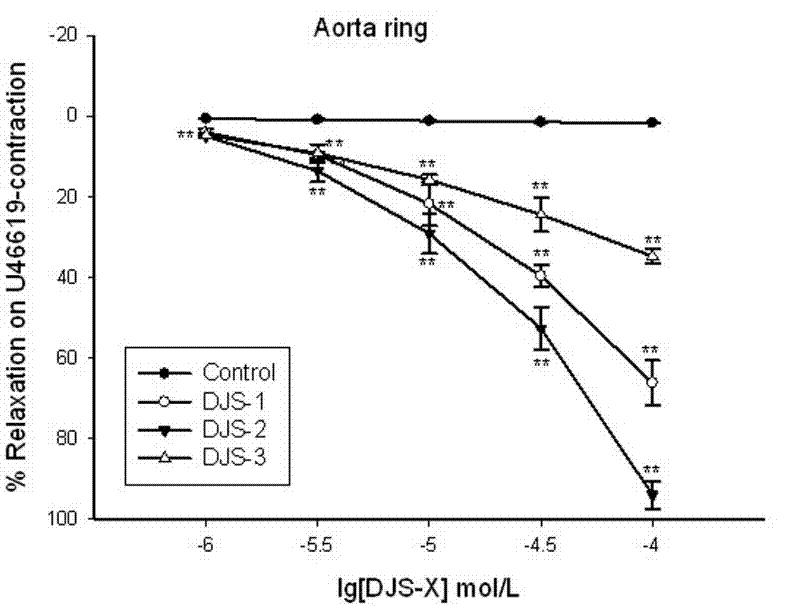
[0031] 1. Dihydroflavonoid novel compound (DJS-X) relaxes the isolated aortic ring in rats
[0032] 1.1 Experimental animals
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses new medicinal application of derivatives of flavanone compound farrerol, a pharmaceutically-acceptable salt of the flavanone compound farrerol and farrerol and the pharmaceutically-acceptable salts of the derivatives, particularly application to preparation of a medicine for treating heart cerebrovascular disease caused by vasoconstriction. Through the experiment of an isolated rat aortic vascular ring, the derivatives and the pharmaceutically-acceptable salts thereof have obvious vasodilation effects and simultaneously have the characteristics of low toxicity, small side effect and the like, can be industrially synthesized, are low in price, and can be matched with the pharmaceutically-acceptable medicinal auxiliary materials for use to be prepared into common preparations such as injection, tablets, pills or capsules and the like which can be clinically used for preventing and controlling heart cerebrovascular disease caused by vasoconstriction, so that new methods and means are provided for clinical treatment of heart cerebrovascular disease.
Description
Technical field [0001] Background technique [0002] [0003] 2 [0004] [0005] Invention content [0006] As well as As well as Essence [0007] [0008] To. [0009] [0010] [0011] [0012] [0013] [0014] [0015]Among them, the medicinal salt (DJS-2, DJS-3, DJS-4) of the medicinal salt (DJS-1) of the dihydroflavonoids (DJS-1) of the dihydroflavonoids (DJS-1) of the medicinal salt (DJS-2, DJS-3, DJS-4) is the bookThe preparation methods are well known by the field of domains: separate compounds 5,7-dihydroxyl-2- (4′-hydroxylphenyl) -6,8-dihydrogen benzene and dihydrous pyra-4-ketone(DJS-1); 5,7-dihydroxyl-2- (2′-nitrate phenyl) -6,8-dihydrogen pyrine pyra-4-ketone (DJS-2); 2-(Fu Fu-2-Bidth) -5,7-two-hydroxyl-6,8-dihydrogen pyrine pyra-4-ketone (DJS-3) and 5,7-dihydroxyl-2- (2 ′, 4'-dichlorophenyl) -6,8-dihydrophoherene-4-ketone (DJS-4), add an appropriate amount of alkaline solution (such as NaOH, KOH solution), and after fully reacting, Extract, add...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/352A61P9/08
Inventor 李青山秦小江韩玲革侯晓敏班树荣石磊
Owner SHANXI MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com