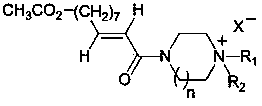
Novel piperazine and homopiperazine derivative and preparation method and use thereof
A technology of homopiperazine and piperazine, which is applied in drug combination, nervous system diseases, organic chemistry, etc., and can solve the problems of limited clinical application and poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Preparation of 1-methyl-4-(10-acetoxy-2-decenoyl)piperazine (compound 1)
[0110] Step 1: the preparation of 10-acetoxy-2-decenoic acid
[0111]
[0112] Under stirring conditions, 10-hydroxy-2-decenoic acid (9.30g, 50mmol) and acetic anhydride (9.43mL, 100mmol) were successively added to a 50mL dry round bottom flask, and the above mixture was placed in a 100°C oil bath Heat to reflux for 1h. The reaction solution was concentrated under reduced pressure, and the crude product obtained was subjected to silica gel column chromatography (eluent: V 丙酮 :V 石油醚 =1:20) separation and purification to obtain 9.51g light yellow oily liquid, productive rate 83.4%.
[0113] Step 2: Preparation of 1-methyl-4-(10-acetoxy-2-decenoyl)piperazine (compound 1)
[0114]
[0115] 10-Acetoxy-2-decenoic acid (2.28g, 10mmol), dichloromethane (30mL) were added to a 50mL round bottom flask, and SOCl was added dropwise under stirring 2 (1.46mL, 20mmol), stirred at room tempe...
Embodiment 2
[0116] Example 2: Preparation of 1-ethyl-4-(10-acetoxy-2-decenoyl)piperazine (compound 2)
[0117]
[0118] The method is the same as that of the intermediate example 1, and ethylpiperazine is substituted for methylpiperazine to obtain 5.56 g of light yellow oily liquid with a yield of 85.8%. 1H NMR (CD 3 OD,500MHz)δ:6.86~6.70(m,1H,CH=CHCO),6.45(d,J=14.9Hz,1H,CH=CHCO),4.07(t,J=6.7Hz,2H,OCH 2 –(CH 2 ) 5 –CH 2 ),3.68(t,J=5.0Hz,4H,(CH 2 ) 2 NC 2 h 5 ),2.55~2.45(m,6H,CON(CH 2 ) 2 and NCH 2 CH 3 ),2.30~2.24(m,2H,OCH 2 –(CH 2 ) 5 –CH 2 ),2.05(s,3H,CH 3 CO 2 ),1.68~1.35(m,10H,OCH 2 –(CH 2 ) 5 –CH 2 ),1.14(t,J=7.2Hz,3H,NCH 2 CH 3 ); 13 CNMR (CD 3 OD,125MHz)δ:171.6,166.3,146.8,119.9,64.3,52.7,52.1,51.8,45.2,41.5,32.1,28.8,28.7,28.6,28.3,25.6,19.7,10.7; ESI-MS m / z:325.23 (M+1) + ;IR(KBr,cm –1 )ν: 1738(C=O), 1659(C=O), 1622(C=C), 1243(C–O–C).
Embodiment 3
[0119] Example 3: Preparation of 1-hexyl-4-(10-acetoxy-2-decenoyl)piperazine (compound 3)
[0120]
[0121] Method is with intermediate embodiment 1, and hexylpiperazine is replaced methylpiperazine, and the thick product of gained is with silica gel column chromatography (eluent: V 甲醇 :V 乙酸乙酯 =1:2) separation and purification to obtain 5.92 g of a light yellow oily liquid, with a yield of 77.8%. 1 H NMR (DMSO-d 6 ,500MHz)δ:6.69~6.10(m,1H,CH=CHCO),6.44(d,J=15.0Hz,1H,CH=CHCO),4.06(t,J=6.6Hz,2H,OCH 2 –(CH 2 ) 5 –CH 2 ),3.55~3.45(m,4H,(CH 2 ) 2 N(CH 2 ) 5 CH 3 ),2.35~2.23(m,6H,CON(CH 2 ) 2 and NCH 2 –(CH 2 ) 4 –CH 3 ),2.21~2.14(m,2H,OCH 2 –(CH 2 ) 5 –CH 2 ),2.00(s,3H,CH 3 CO 2 ),1.60~1.22(m,18H,OCH 2 –(CH 2 ) 5 –CH 2 and NCH 2 –(CH 2 ) 4 –CH 3 ),0.87(t,J=6.5Hz,3H,NCH 2 –(CH 2 ) 4 –CH 3 ); 13 C NMR (DMSO-d 6 ,125MHz)δ:170.8,164.8,145.6,121.1,64.2,64.1,58.2,53.7,53.0,45.5,32.0,31.7,30.4,28.9,28.8,28.6,28.3,27.8,27.0,26.6,25.8,22.5,21.2 ,14.3;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com