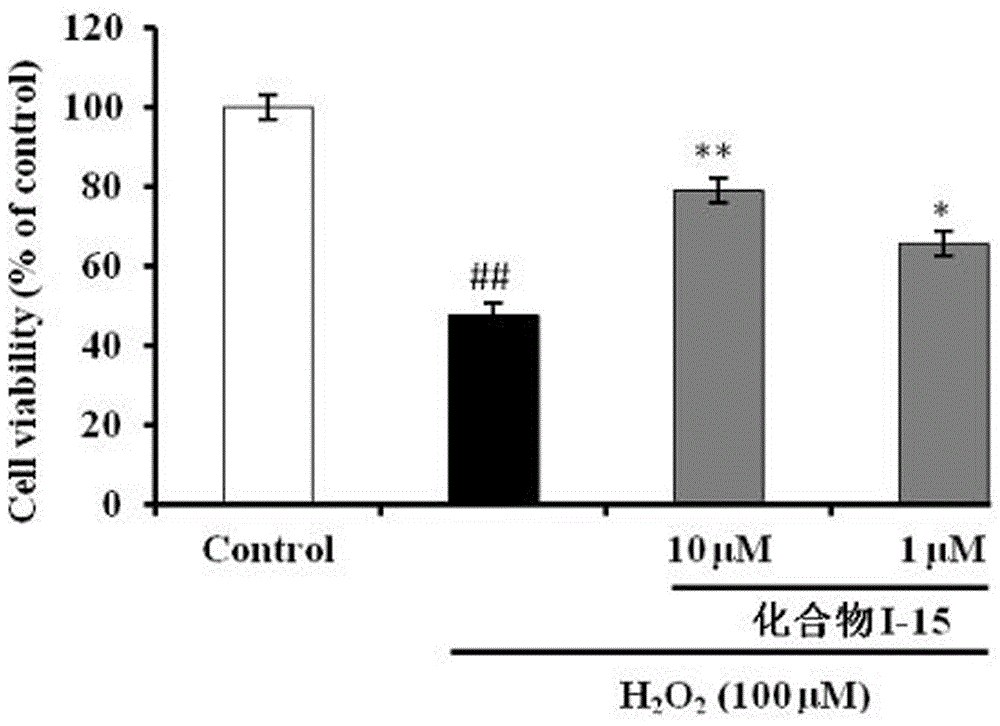
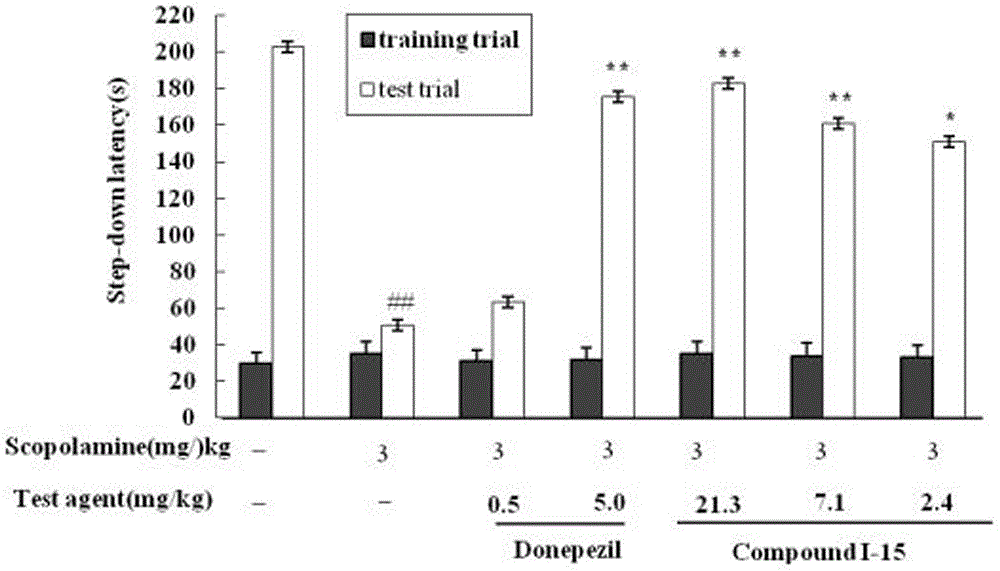
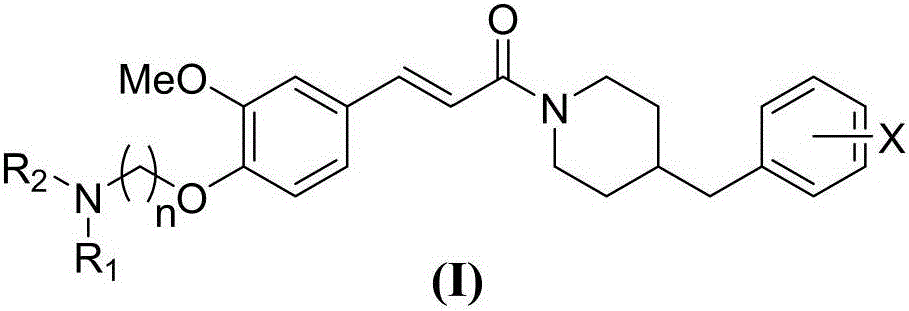
N-(benzylpiperidyl)-feruloylagmatine-O-alkylamine compound, preparation method and application thereof
A technology of benzyl piperidine and ferulamide, applied in the field of medicinal chemistry, can solve problems such as deterioration, dementia, neuron loss, etc., and achieve the effects of low toxicity, improvement of memory reproduction dysfunction, and good clinical application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of preparation method of N-(benzylpiperidinyl)-ferulamide-O-alkylamine compound, its chemical reaction equation is as follows:
[0046]
[0047] Including the following steps, specifically X, n and NR 1 R 2 See Table 1, and the specific parameters are shown in Table 2:
[0048] The first step: add ferulic acid 1 (150mg, 0.772mmol), EDCI (3.54g, 0.926mmol), HOBT (178mg, 0.926mmol) and dichloromethane into the reaction flask, stir well and add benzylpiperidine Compound 2 (0.926mmol) was added, stirred at room temperature overnight, and monitored by TLC; after the reaction, the solvent was evaporated under reduced pressure, water (40mL) was added to the residue, extracted with dichloromethane (40mL×2), and the organic layers were combined Afterwards, it was washed with saturated aqueous sodium chloride solution (40 mL), dried over anhydrous sodium sulfate, filtered, the filtrate was evaporated to remove the solvent under reduced pressure, and the residue was pu...
Embodiment 2-90
[0052] Embodiments 2-90 are all prepared according to the method of Example 1. The chemical structures of the target products in Table 1 have been verified. 1 H NMR, 13 Confirmed by C NMR and ESI-MS.
[0053] Table 1 N-(benzylpiperidinyl)-ferulamide-O-alkylamines of Examples 1-90 of the present invention
[0054]
[0055]
[0056]
[0057] Part target compound N-(benzylpiperidinyl)-ferulamide-O-alkylamine compound in table 1 prepared by the present invention 1 HNMR, 13 C NMR and ESI-MS data are as follows:
[0058] The preparation of the target compound I-8, the operation process is the same as in Example 1, and a light yellow oily substance is obtained, with a yield of 59.2%. 1 HNMR (400MHz, CDCl 3 )δ7.60(d, J=15.6Hz, 1H, C=CH), 7.33-7.17(m, 8H, 8×Ar-H), 7.13(d, J=7.6Hz, 2H, 2×Ar-H ),7.07(d,J=8.0Hz,1H,Ar-H),7.03(s,1H,Ar-H),6.86(d,J=8.4Hz,1H,Ar-H),6.76(d,J =15.2Hz, 1H, C=CH), 4.69(t, J=6.0Hz, 1H, 1 / 2phCH 2 ), 4.08(t, J=6.0Hz, 3H, 1 / 2phCH 2 +OCH 2 ),3.86(s,3H...
Embodiment 91-95
[0075] Examples 91-95 used the same method as Example 1 to prepare compound I-1 in Table 1, the difference being that the parameters used in the preparation method were different, specifically as shown in Table 2, wherein the first in Table 2 The mol ratio in the step is the molar feed ratio of ferulic acid, benzylpiperidine compound and condensation agent, the mol ratio in the second step is the molar feed ratio of intermediate compound, dibromoalkane and alkali, in the 3rd step The molar ratio is the molar feed ratio of bromine compound intermediate, secondary amine and alkali.
[0076] Table 2 embodiment 1 and embodiment 91-95 parameter data
[0077]
[0078]
[0079] As can be seen from Table 2, using different parameters to prepare the compound I-1 in Table 1, the yield of the product is 25-90%, and the yield of the target product prepared by the parameters in Example 91 is the highest, illustrating that the present invention is implemented The parameters in Exampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com