Compound 1,10-N-decylene lycorine dibromo salt, pharmaceutical composition and application thereof in medicine
A technology of decylene double stones and compounds, which is applied in the field of medicine and can solve problems such as compounds that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
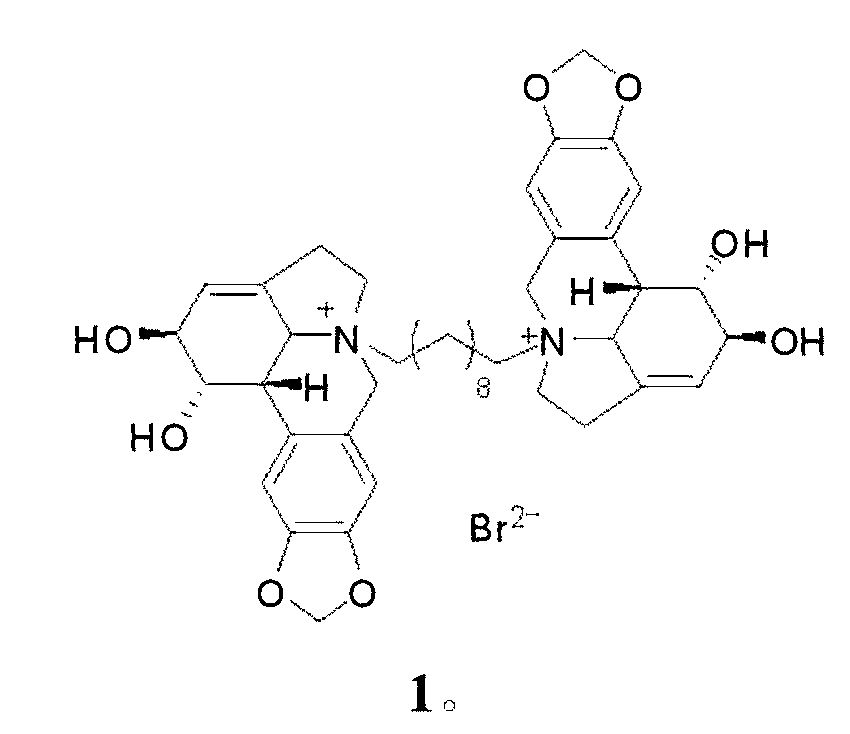
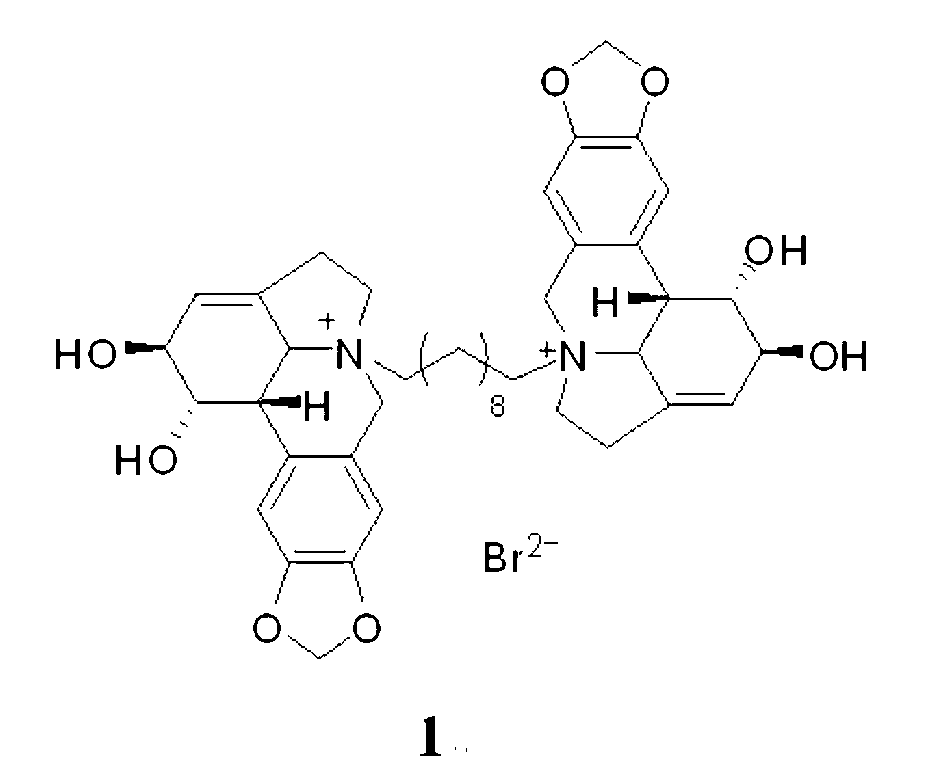
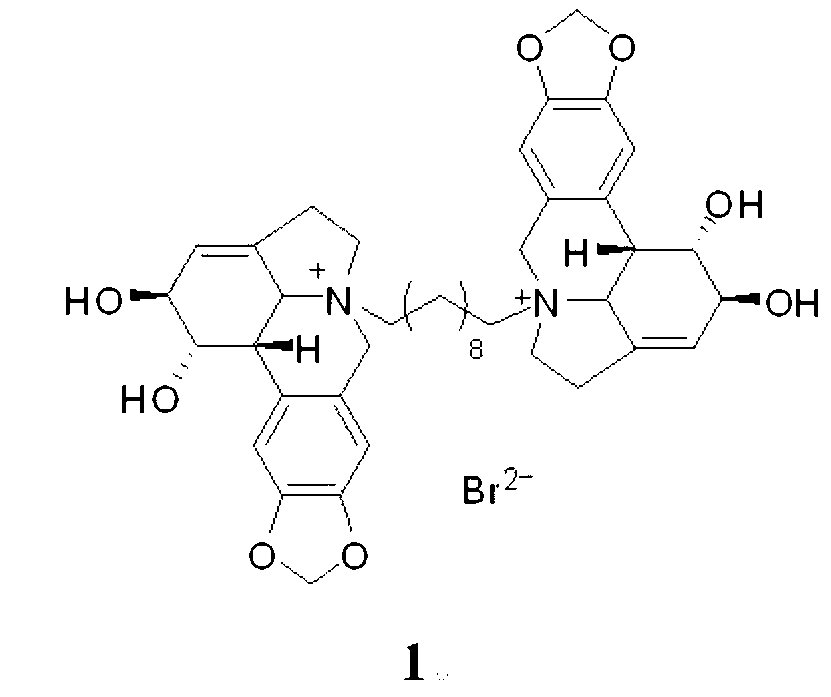
[0027] Compound 1, the preparation of 10-N-decylene bislycorine dibromide, namely compound 1:
[0028] Take lycorine (144 mg, 0.5 mmol), add 1 mL of dry DMF, 1,10-dibromodecane (75 mg, 0.25 mmol). After mixing, stir at 100 oC for 6-12 h under nitrogen protection. The solvent in the reaction system was evaporated to dryness, using gel LH-20 chromatographic column chromatography, methanol or water as the eluent, to obtain compound 1, 10-N-decylidene dilycorine dibromide, compound 1 (131 mg , 0.125 mmol, 60% yield).
[0029] Compound 1, 10-N-decyl dilycorine dibromide salt is compound 1, light yellow solid; 1 H NMR (CD 3 OD, 500 MHz) δ 7.11 (s, 2H), 7.08 (s, 2H), 6.05 (s, 4H), 5.80 (s, 2H), 4.90 (d, J = 14.0 Hz, 2H), 4.61 (br. s, 2H), 4.26 (br. s, 2H), 4.27 (d, J = 14.0 Hz, 2H), 3.90 (d, J = 10.0 Hz, 2H), 3.63 (m, 2H), 2.87-3.25 (m , 10H), 1.77 (m, 4H), 1.30 (m, 12H); ESIMS m / z: 357 [M] 2+ , 793 [M+Br] + .
[0030] Compound 1, the structural formula of compound 1, 10-N-de...
Embodiment 2
[0035] Determination of the cholinesterase inhibitory activity of compound 1, 10-N-decyldilycorine dibromide of the present invention, that is, compound 1:
[0036] For the assay method of human acetylcholinesterase (hAChE) and butyrylcholinesterase (hBChE) inhibitory activity, see Ellman et al [Ellman GL, Courtney KD, Andres V, Jr., Feather-Stone RM: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical pharmacology 1961, 7:88-95.], the activity results of compound 1 are shown in Table 1.
[0037] Table 1 Inhibitory effect of compound 1 prepared in Example 1 on human acetylcholinesterase (hAChE) and butyrylcholinesterase (hBChE)
[0038]
[0039] a Mean ± SD; n = 3
Embodiment 3
[0041] Tablet: Compound 1 obtained in Example 1 10 mg, lactose 180 mg, starch 55 mg, magnesium stearate 5 mg;
[0042]Preparation method: mix compound 1 obtained in Example 1 with lactose and starch, wet it evenly with water, sieve the wet mixture and dry it, then sieve it, add magnesium stearate, and press the mixture into tablets, each weighing 250 mg, the compound content is 10 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com